Trans fatty acids influence the oxidation of LDL in ECV304 cells
Abstract
The objectives of this study are to explore whether separate sources of trans fatty acids (TFA) have different effects on ECV304 cell line and to further elucidate the oxidation mechanism induced by TFA. ECV304 cells are used in the study because they display many endothelial features. Cell apoptosis rates increased in a dose-dependent manner following 24-h treatment with TFA from separate sources. Additionally, TFA stimulated human alpha-defensin 1 (HNP-1) expression and resulted in a significant increase in both malondialdehyde (MDA) and ROS levels. MDA levels reach their peak at 18 h. HNP-1 expression levels increase at 2 h and then reach their peak at 10 h. At the same time, the protein carbonyl (PCO) value declines slightly. After 10 h of TFA co-culture, the cells were washed and fresh low-density lipoprotein (LDL) was added. MDA generation significantly increased after 6 h and it could be inhibited by 4-aminobenzoic acid hydrazide (ABAH) or sodium ferulate. However, after the TFA co-culture for 2 h, adding LDL for 6 h just caused slight MDA generation change and the MDA generation could be inhibited by verapamil or sodium ferulate. TFA from different sources did not have different effects on ECV304. HNP-1 mediates the oxidation induced by TFA by activating ROS. Furthermore, TFA can stimulate the oxidation of LDL in ECV304 cells through both passive and active pathway. In the oxidation induced by linoelaidic acid, ABAH can decrease the MDA generation in active oxidation pathway and verapamil can decrease the MDA generation in passive oxidation pathway.
Abbreviations:
ABAH, 4-aminobenzoic acid hydrazide; CHD, coronary heart disease; HNP-1, human α-defensin 1; LDL, low-density lipoprotein; MPO, myeloperoxidase; PCO, protein carbonyl; TFA, trans fatty acid; TMB, tetramethylbenzidine
Introduction
Trans fatty acids (TFAs) refer to unsaturated fatty acids with at least one double bond in the trans configuration. TFAs are formed during the production of vegetable oils which are partially hydrogenated in order to keep the oils stable and prevent the development of bad taste 1. TFA are also found in dairy products that come from the biohydrogenation in the alimentary canal of ruminant animals 2. TFA formed via industrial hydrogenation are widely considered to raise the ratio of low-density lipoprotein (LDL) to high density lipoprotein (HDL) 3. As a result, TFA are considered to be an independent factor of coronary heart disease (CHD) and sudden death 4-7. Most of the research today focuses on the influence of TFA on the concentration of lipoprotein in the blood. However, the metabolism of lipoprotein by TFA is still not clear. Kummerow reported that oxidation of cholesterol in LDL may be involved in the CHD caused by TFA 8.
Atherosclerosis is a progressive inflammatory disease 9. Endothelial cells play a vital role in the process of atherogenesis 10, and they provide a barrier to protect against the formation of thrombi and inflammation 11. It is widely known that endothelial cell dysfunction contributes to the occurrence of atherosclerosis. According to 12, certain TFAs induce cell apoptosis.
Clinical and epidemiological studies reveal that increased levels of LDL cholesterol promote premature atherosclerosis. According to the oxidative modification hypothesis, LDL is not atherogenic in its native state 13. In contrast, the oxidized form of LDL predicts an early event in atherosclerosis, and it is this oxidized version of LDL that contributes to atherogenesis 14. Based on our earlier experiments, cells may oxidize LDL either by passive or active oxidation. Cell oxidative stress caused by microbial infection activates LDL oxidation to normal levels; this is referred to as passive oxidation. When cell oxidative stress is activated by high-level LDL this is referred to as active LDL oxidation 15.
Human α-defensin 1 (HNP-1) is a small, cationic, antimicrobial peptide that can be found in mammals, insects, and plants. It constitutes greater than 5% of the total protein in human neutrophils. Classically, it is considered to be antibiotic. In the past few years, researchers have found that HNP-1 is associated with the occurrence of cardiovascular disease, especially in the LDL oxidation process. Moreover, our previous study using a real-time RT-PCR-based assay demonstrated that HNP-1 is involved in the oxidation of LDL 16. Moreover, HNP-1 participates in the two oxidation pathways in different manners. Although mRNA expression increases in both passive and active pathways, HNP-1 plays a more significant role in promoting oxidation in the active pathway. However, the specific mechanisms are still not clear. According to published data, ECV304 cells express many endothelial characteristics. Many endothelial markers (von Willebrand factor, uptake of LDL, vimentin) could clearly be identified in ECV304. So in this study, ECV304 cells are continuous to be selected to explore oxidation environment in cells 17.
The purpose of our study is to verify whether separate sources of TFA have different effects on ECV304 and whether the adverse effect of TFA on CHD is partially caused by their effects on apoptosis of endothelial cells and the oxidative stress. Furthermore, the specific mechanism of oxidative stress caused by TFA in ECV304 cells is also explored. Additionally, we assess the roles of active and passive oxidation pathways in the process of LDL oxidation induced by TFA in ECV304 and explore the difference between them.
Materials and methods
Materials
ECV304 cells were purchased from China Center for culture collection. TFAs were purchased from Nu-Chek Prep Incorporated (Elysian, MN, USA). Annexin V: FITC and propidium iodide were purchased from Invitrogen (Eugene, Oregon, USA). The MDA detection kit was purchased from the Nanjing Jiancheng Incorporated (Nanjing, China). The H2DCFDA was acquired from Sigma–Aldrich (St Louis, MO, USA).
Methods
ECV304 culture and treatment
ECV304 was maintained in tissue culture flasks with DMEM containing 10% fetal bovine serum and the bullet kit materials as specified by the manufacturer. Cells were maintained at 37°C in a humidified atmosphere in the presence of 5% CO2. The medium was replaced every 2 days until cells were confluent.
Annexin V and propidium iodide staining
Digest cells and change to 6-well tissue culture plates with DMEM containing 10% fetal bovine serum and the bullet kit materials. After reaching 80% confluence, the medium was changed and the cells were treated for 24 h with 0.02, 0.2, and 1.0 mmol/L elaidic acid, trans vaccenic acid, or linoelaidic. Then the cells were harvested from the wells by trypsin digestion.
The effects of TFAs on ECV304 were measured by dual staining with Annexin V: FITC and propidium iodide, using the Apoptosis Detection Kit from Invitrogen Incorporated. Annexin V and propidium iodide was added to the cellular suspension according to the manufacturer's instructions, and the sample fluorescence of cells was analyzed by flow cytometry (Beckman Coulter, USA).
Cells that were Annexin V: FITC positive and PI negative were identified as early apoptotic. Cells which were Annexin V: FITC positive and PI positive were identified as cells in late apoptosis or necrotic.
MDA detection
The oxidative stress levels were determined by MDA detection Kit (Nanjing Jiancheng). We treated the ECV304 with medium alone or 1 mmol/L linoelaidic acid, and then took the supernatant every 2 h for 24 h to test the MDA content according to the manufacturer's.
Real time PCR analysis of HNP-1 expression
Treat the ECV304 with medium alone or 1 mmol/L linoelaidic acid for 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24 h and total RNA was isolated with Trizol reagents (Invitrogen Incorporated, San Diego, CA, USA) according to the protocol from the manufacturer. The mRNA levels of HNP-1 in the cells were determined by a SYBR Green-based real time RT-PCR 15.
Protein carbonyl levels (PCO detection)
Treat the ECV304 with medium alone or 1 mmol/L linoelaidic acid for 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24 h. The content of carbonyl in oxidatively modified proteins was measured by the method of Levine et al. 18. The quantification of hydrazones formed by the reaction of carbonyl groups with 2,4-dinitro-phenylhy-drazine (DNPH) reflects the oxidative damage to proteins inside the cells. Briefly, 0.1 mL of serum was incubated with 1.0 mL of 20 mM DNPH solution (dissolved in 2 mol/L HCL) in the dark for 60 min. Add 500 µL 20% v/v trichloroacetic to the solution and place it at RT for 5–10 min. Then, centrifuge the solution, and the proteins were precipitated in the bottom. Remove the supernatant and wash the sediment three times with ethanol and ethyl acetate. Then re-suspend the sediment in 500 µL of 6 M guanidine and read the absorbance at 370 nm.
Effects of ECV304 on LDL oxidation induced by trans fatty acid
To explore the oxidation pathway of LDL induced by TFAs, two groups of experiments were designed. In the first group, incubate ECV304 with 1 mmol/L linoelaidic acid for 2 h and then rinse three times with phosphate buffer solution (PBS). Added 100 µg/mL LDL, test the content of MDA in the medium 6 h later. In the other group, treat the ECV304 with linoelaidic acid for 10 h and then rinse three times with PBS. Added 100 µg/mL LDL, test the MDA level in medium after 6 h.
Reactive oxygen species (ROS) production test
Culture the ECV304 in 6-well tissue culture plates with DMEM containing 10% fetal bovine serum. The medium was replaced every two days until 90% confluent. After treating the ECV304 with medium alone or 1 mmol/L linoelaidic acid for 1 min, 5 min, 10 min, 30 min, 2 h, or 10 h, wash the cells with PBS three times and add fresh LDL to incubate the cells for 6 h. Then, replace the medium and add H2DCFDA at a concentration of 10 µmol/L. After incubating the cells free from light for 30 min, remove the medium and digest the cells, then measure the content of ROS by flow cytometry.
Myeloperoxidase (MPO) activity test
The MPO activity was determined by using a noncarcinogenic compound, tetramethylbenzidine (TMB). For the determination of specific activity, the reaction was carried out at RT in 50 mM sodium acetate buffer, pH 4.5, 0.88 mM TMB, and 5 mM H2O2. The reaction was initiated by the addition of enzyme, and the accumulation of oxidized TMB was recorded with OD measurement at 655 nm wave length. One unit of activity is defined as the amount of enzyme necessary to decompose 1 µmol of H2O2 per minute.
Results
Trans fatty acids induce apoptosis of ECV304
After treating ECV304 cells with 0.02, 0.2, or 1 mM elaidic acid, trans vaccenic acid, or linoelaidic acid for 24 h (as described in Section 2), the percent of apoptotic cells was measured by flow cytometry. Early apoptotic cells were Annexin-positive, and late apoptotic cells were stained by propidium iodide.
We found that all three TFAs tested induce both early and late apoptosis in a dose-dependent manner. Incubation of ECV304 cells with 0.02, 0.2, or 1 mM elaidic acid caused an increase in early apoptosis from 9.3 ± 1.4% to 9.7 ± 1.3%, 10.4 ± 1.2%, 15.8 ± 2.7%, 22.3 ± 3.2% and an increase in late apoptosis from 8.3 ± 0.75% to 9 ± 1%, 9.9 ± 2.3%, 10.8 ± 1.9%, 18.8 ± 2.4%. The total percent of apoptotic cells increased from 17.6 ± 1.9% to 18.7 ± 2.1%, 20.3 ± 2.7%, 26.6 ± 3.1%, 41.1 ± 3.8% (see Fig. 1A).
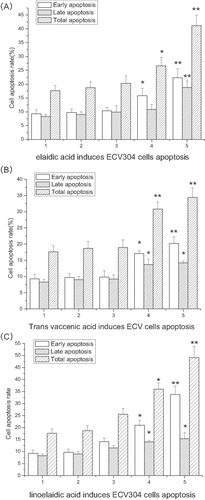
TFAs induce ECV304 cells apoptosis. ECV304 cells were cultured with 0.02, 0.2, or 1 mmol/L elaidic acid (Fig. 1A), trans vaccenic acid (Fig. 1B), or linoelaidic acid (Fig. 1C) for 24 h. Cell apoptosis rates were measured by flow cytometry. The early apoptosis rate, late apoptosis, and total apoptosis rates were presented in the figure. Group 1: ECV304 was treated with medium only for 24 h; Group 2: ECV304 is treated with 0.1% ethanol for 24 h; Group 3: ECV304 was treated with 0.02 mmol/L elaidic acid (Fig. 1A), trans vaccenic acid (Fig. 1B), or linoelaidic acid (Fig. 1C) for 24 h; Group 4: ECV304 was treated with 0.2 mmol/L elaidic acid (Fig. 1A), trans vaccenic acid (Fig. 1B), or linoelaidic acid (Fig. 1C) for 24 h; Group 5: ECV304 is treated with 1 mmol/L elaidic acid (Fig. 1A), trans vaccenic acid (Fig. 1B), or linoelaidic acid (Fig. 1C) for 24 h. The results represent the mean value of three independent experiments. (n = 3, *p<0.05, **p<0.01, mean ± S.D.)
Similarly, incubation of ECV304 with 0.02, 0.2, or 1 mM trans vaccenic acid for 24 h caused an increase in early apoptosis from 9.3 ± 1.4% to 9.7 ± 1.3%, 9.8 ± 2%, 17.1 ± 0.9%, 20.2 ± 2.1% and an increase in late apoptosis from 8.3 ± 0.75% to 9 ± 1%, 9.2 ± 1.3%, 13.7 ± 1.7%, 14.2 ± 0.6%. Consequently, the total apoptotic rate increased from 17.6 ± 1.9% to 18.7 ± 2.1%, 19.0 ± 2.3%, 30.8 ± 2.2%, 34.4 ± 2.9% (see Fig. 1B).
Incubation of ECV304 with 0.02, 0.2, 1 mmol/L linoelaidic acid caused an increase in early apoptosis from 9.3 ± 1.4% to 9.7 ± 1.3%, 14.1 ± 1.5%, 21 ± 1.9%, 33.7 ± 3.6% and an increase in late apoptosis from 8.3 ± 0.75% to 9 ± 1%, 11.5 ± 1%, 14 ± 0.7%, 15.4 ± 2.5%. The sum of apoptotic cells increased from 17.6 ± 1.9% to 18.7 ± 2.1%, 25.6 ± 2.3%, 36 ± 2.6%, and 49.1 ± 4.7% (see Fig. 1C).
24 h gene expression profiles of ECV304 cells induced by trans fatty acids
The effects of 1 mmol/L TFAs on the expression of HNP-1 mRNA are presented in Fig. 2. HNP-1 mRNA expression level significantly increased with 1 mmol/L elaidic acid (treatment/ethanol, 1.23697 ± 0.03183), trans vaccenic acid (treatment/ethanol, 1.2187 ± 0.11358), and linoelaidic acid (treatment/ethanol, 1.41653 ± 0.14774) treatments for 24 h when compared to control cells treated with ethanol. The three TFA did not significantly differently regulate HNP-1 mRNA expression. However, linoelaidic acid did increase HNP-1 mRNA levels to a slightly higher level (see Fig. 2).
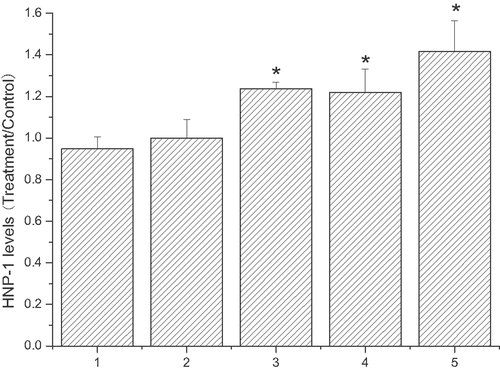
HNP-1 mRNA expression of ECV304 cells induced by 1 mmol/L TFA. ECV 304 cells were treated under different conditions and then mRNA levels of HNP-1 were determined by real time RT-PCR. Column 1: ECV304 was treated with medium only for 24 h; Column 2: ECV304 was treated with 0.1% ethanol for 24 h; Column 3: ECV304 was treated with 1 mmol/L elaidic acid for 24 h; Group 4: ECV304 was treated with 1 mmol/L trans vaccenic acid for 24 h; Group 5: ECV304 was treated with 1 mmol/L linoelaidic acid for 24 h. The results represent the mean value of three independent experiments. (n = 3, *p<0.05 compared with control, mean ± S.D.)
Linoelaidic acid stimulates MDA generation in ECV304
The time course of MDA generation is presented in Fig. 3. After incubation of ECV304 with 1 mmol/L linoelaidic acid or ethanol for different time periods, the content of MDA in the medium increased. As shown in Fig. 3, the concentration of MDA reaches its peak at 18 h.
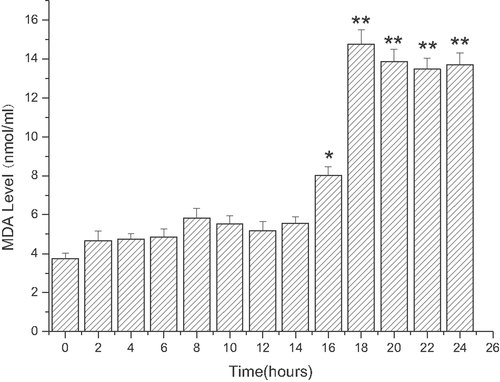
MDA generation induced by linoelaidic acid in ECV304 at different time points: The concentration of MDA (nmol/mL) in the medium was detected after ECV304 cells were incubated with linoelaidic acid for different periods. From Column 1 to Column 13, ECV and Linoelaidic acid were co-cultured respectively for 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24 h. The results represent the mean value of three independent experiments. (n = 3, *p<0.05, **p<0.01, compared with control, mean ± S.D.)
Time course of HNP-1 mRNA expression
The expression of HNP-1 mRNA at different time points is presented in Fig. 4. Compared to the control group, the expression of HNP-1 mRNA increased over time. It was significantly elevated at 2 h ((treatment(HNP-1/b-actin)/enthanol(HNP-1/b-actin)), 1.9656 ± 0.10435) and reached its peak at 10 h ((treatment(HNP-1/b-actin)/enthanol(HNP-1/b-actin)), 4.23479 ± 0.20192) (see Fig. 4).
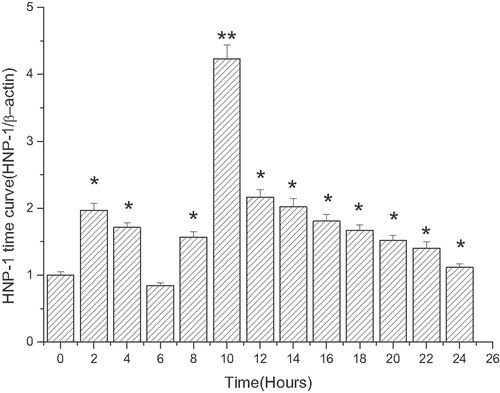
HNP-1 mRNA expression time course induced by linoelaidic acid. ECV304 cells were incubated with linoelaidic acid for different periods. From Column 1 to Column 13, ECV 304 cells and linoelaidic acid were co-cultured, respectively, for 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24 h. Then, the mRNA expression levels of HNP-1 were determined by real time RT-PCR. The results represent the mean value of three independent experiments. (n = 3, *p<0.05, **p<0.01, compared with control, mean ± S.D.)
Time course of protein carbonyl (PCO) levels in ECV304 induced by linoelaidic acid
The content of PCO in the medium is shown in Fig. 5. The quantification of carbonyl groups in the proteins decreases slightly after treatment with 1 mmol/L linoelaidic acid over time. Although, compared to the control group, there was a trend toward a slow decrease over time, the difference was not very significant (see Fig. 5).
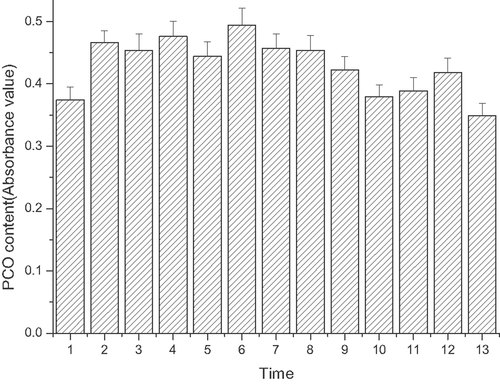
Time course of PCO levels induced by linoelaidic acid. ECV304 cells were incubated with linoelaidic acid for different periods. From Column 1 to Column 13, ECV and Linoelaidic acid were co-cultured for 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24 h. The concentration of PCO was tested by the assay discussed in Section 2. The results represent the mean value of three independent experiments. (n = 3, *p<0.05 compared with control, mean ± S.D.)
ROS levels in ECV304 cells
Fluorescence intensity measured by flow cytometry is indicative of the ROS levels in cells induced by 1 mmol/L linoelaidic acid, and this is presented in Fig. 6. From the figure, it is clear that there is no significant change in ROS levels at 1 min (11.3 ± 1.673), 10 min (10.4 ± 1.545), or 30 min (11 ± 1.463). However, at 2 h (18.4 ± 1.997) there is an obvious increase and at 10 h (86.9 ± 6.487), it reaches its peak (see Fig. 6).
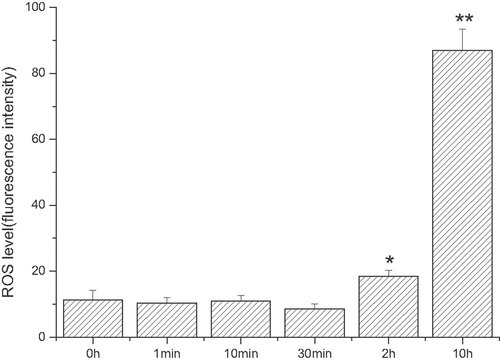
ROS level induced by linoelaidic acid in ECV304. ECV304 cells were incubated with linoelaidic acid for different time. Then, ROS content in cells were detected by flow cytometry. From Column 1 to Column 13, ECV304 and linoelaidic acid were co-cultured for 1 min, 10 min, 30 min, 2 h, or 10 h. The results represent the mean value of three independent experiments. (n = 3, *p<0.05, **p<0.01, compared with control, mean ± S.D.)
LDL oxidation induced by TFA
The oxidation of LDL induced by TFA was measured by MDA generation. After treatment with linoelaidic acid for 10 h and LDL for 6 h, the MDA concentration in the medium is much higher (63.96588 ± 3.37265 nmol/mL) compared to the control group (40.79254 ± 1.96253 nmol/mL), which was treated with linoelaidic acid for 16 h. However, in the other group, there is no significant distinction between the treatment (47.07267 ± 2.47398 nmol/mL) group which is co-cultured LDL for 6 h followed by linoelaidic acid for 2 h and control group (48.5992 ± 2.76587 nmol/mL) which is treated with linoelaidic acid for 8 h (see Fig. 7).
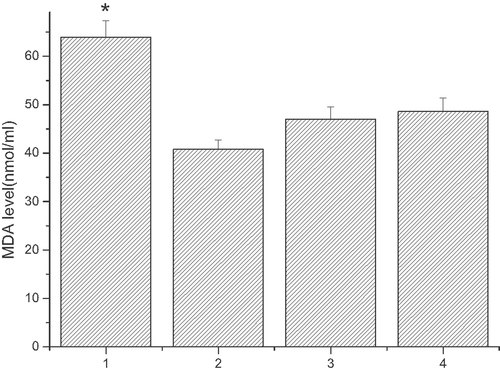
MDA level induced by linoelaidic acid in different conditions. ECV304 cells were treated with different conditions. Column 1, adding fresh LDL for 6 h after linoelaidic acid co-culture 10 h; Column 2, linoelaidic acid co-culture 16 h; Column 3, adding fresh LDL for 6 h after the linoelaidic acid co-culture 3 h; Column 4, linoelaidic acid co-culture 8 h. The results represent the mean value of three independent experiments. (n = 3, *p<0.05 compared with control, mean ± S.D.)
MDA generation inhibited by sodium ferulate
After co-incubate with linoelaidic acid for 2 or 10 h, cells were treated with 100 µg/mL LDL and 100 µmol/L sodium ferulate for 6 h. As shown in Fig. 8, in both groups, MDA generations were significantly inhibited when sodium ferulate was added. In the first group, MDA generation decreased from 12.58903 ± 1.65732 to 5.98745 ± 2.12416 nmol/mL; in the second group, MDA generation decreased from 21.57152 ± 0.91846 to 10.25715 ± 0.51348 nmol/mL.
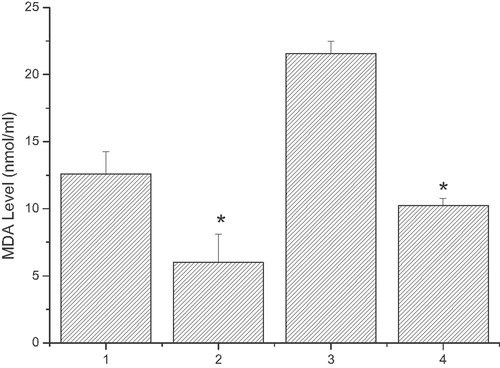
MDA generation inhibited by sodium ferulate. ECV304 cells were treated with different conditions. Column 1, adding fresh LDL for 6 h after linoelaidic acid co-culture 2 h; Column 2, adding fresh LDL and sodium ferulate for 6 h after linoelaidic acid co-culture 2 h; Column 3, adding fresh LDL for 6 h after the linoelaidic acid co-culture 10 h; Column 4, adding fresh LDL and sodium ferulate for 6 h after the linoelaidic acid co-culture 10 h. The results represent the mean value of three independent experiments. (n = 3, *p<0.05 compared with control, mean ± S.D.)
Myeloperoxidase (MPO) activity and MDA generation inhibited by 4-aminobenzoic acid hydrazide (ABAH) and verpamil
After co-incubate with linoelaidic acid for 2 h, cells were treated with 100 µg/mL LDL and 100 µmol/L ABAH, 100 µmol/L verapamil for 6 h, then test the MPO activity and MDA generation. The results showed that ABAH did not decrease the MDA generation, but verapamil did. And both ABAH and verapamil inhibited the MPO activity in this group. In the other group, after co-incubate with linoelaidic acid for 10 h, cells were treated with 100 µg/mL LDL and 100 µmol/L ABAH, 100 µmol/L verapamil for 6 h. As shown in the Fig. 9, ABAH significantly inhibited MDA generation but verapamil did not. And both ABAH and verapamil inhibited the MPO activity.
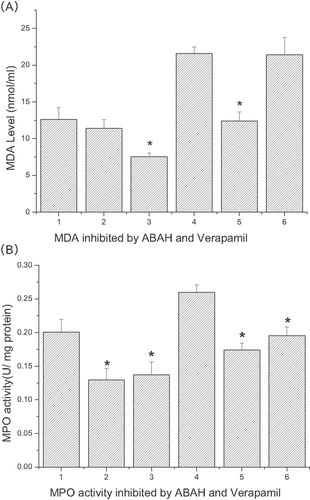
(A) MDA generation inhibited by ABAH and verapamil. ECV304 cells were treated with different conditions. Column 1, adding fresh LDL for 6 h after linoelaidic acid co-culture 2 h; Column 2, adding fresh LDL and ABAH for 6 h after linoelaidic acid co-culture 2 h; Column 3, adding fresh LDL and verapamil for 6 h after linoelaidic acid co-culture 2 h; Column 4, adding fresh LDL for 6 h after the linoelaidic acid co-culture 10 h; Column 5, adding fresh LDL and ABAH for 6 h after the linoelaidic acid co-culture 10 h; Column 6, adding fresh LDL and verapamil for 6 h after the linoelaidic acid co-culture 10 h The results represent the mean value of three independent experiments. (n = 3, *p<0.05 compared with control, mean ± S.D.). (B) MPO activity inhibited by ABAH and verapamil. ECV304 cells were treated with different conditions. Column 1, adding fresh LDL for 6 h after linoelaidic acid co-culture 2 h; Column 2, adding fresh LDL and ABAH for 6 h after linoelaidic acid co-culture 2 h; Column 3, adding fresh LDL and verapamil for 6 h after linoelaidic acid co-culture 2 h; Column 4, adding fresh LDL for 6 h after the linoelaidic acid co-culture 10 h; Column 5, adding fresh LDL and ABAH for 6 h after the linoelaidic acid co-culture 10 h; Column 6, adding fresh LDL and verapamil for 6 h after the linoelaidic acid co-culture 10 h. The results represent the mean value of three independent experiments. (n = 3, *p<0.05 compared with control, mean ± S.D.)
Discussion
There are differing opinions on the effects of different TFA on organisms. A number of studies have shown that all TFA are harmful to the cardiovascular system because they raise the LDL to HDL ratio 3, 19-24, which is believed to be an independent risk factor of cardiovascular disease 25. However, other research finds that some TFA are not harmful to human beings 26. Moreover, certain types of TFA may even have antiatherogenic effects. For example, trans vaccenic acid may fall into this category 27. To explore the possible existence of distinct effects of TFA on ECV304, we chose TFA from various sources. Elaidic acid is a typical industrial TFA, produced by partial hydrogenation of vegetable oil. Vaccenic acid (11-trans-C18:1) is the predominant TFA in milk and meat from ruminant animals. Considering the potential effects of the double linkage numbers on cells, linoelaidic acid is also chosen to be our study object 3. In terms of apoptosis, it is clear that all three kinds of TFA induce both early and late cell apoptosis and there is no obvious distinction among them. Furthermore, all TFA induce apoptosis to similar extents, but linoelaidic acid causes slightly higher levels. For this reason, we chose linoelaidic acid for further study on ECV304. Since the apoptosis rates in the 0.02 mmol/L, 0.2 mmol/L treated groups are not significantly different compared to the control group, we chose 1 mmol/L as the concentration in the following study.
Most current work focuses on the influence of TFAs on the concentration of LDL and HDL in the peripheral blood. However, the mechanism of LDL metabolism 3, 18-23, especially the oxidation of LDL by TFA, is rarely studied. Based on Kummerow's opinion 8, the side effect of TFA on endothelium cells may be related to the oxidation of the fatty acids and oxidation of the cholesterol in the LDL. Our study is the first to discuss the oxidation stress caused by TFA in ECV304 in detail and demonstrate that TFA stimulates the oxidation of LDL. In our previous study, HNP-1 was shown to be involved in the oxidation of LDL. HNP-1 mRNA expression increased significantly in the oxidation process. In order to verify if HNP-1 participates in the process of cell apoptosis and oxidation in cells caused by TFA the HNP-1 level in cells treated with elaidic acid, trans vaccenic acid, and linoelaidic acid for 24 h was tested. We found that the levels of HNP-1 increased (elaidic acid/ethanol: 1.23697; trans vaccenic acid/ethanol: 1.2187; linoelaidic acid/ethanol: 1.41653;), so it is reasonable to hypothesize that HNP-1 may be involved in the oxidative stress in cells induced by TFA. Among the three treatment groups, linoelaidic acid caused the largest increase in HNP-1, which correlates with the results of the cell apoptosis test. Thus, we chose linoelaidic acid for further experiments.
HNP-1 mRNA expression levels are not significantly altered after 24 h of TFA treatment. To further explore the oxidation process, the content of MDA in the medium was tested every 2 h for 24 h following treatment with 1 mmol/L linoelaidic acid. We found that the concentration of MDA increases gradually and then peaks at 18 h. This is in contrast to PCO, which decreases at a slight rate. That is to say, after linoelaidic acid treatment, fats are oxidized while proteins remain stable. It is also reasonable to hypothesize that the TFA itself was oxidized in the medium in ECV304.
To further explore the relationship between HNP-1 and oxidation in cells, a time course of mRNA expression of HNP-1 was performed. From these results, we can find that it rises similar to the increase of MDA. According to our work and the work of others, HNP-1 participates in the LDL oxidation process in ECV304. In our prior studies, HNP-1 reaches its peak a few hours before MDA peaks, and this is what we saw in our current study as well. Based on this, it is likely that HNP-1 participates in oxidation in TFA-treated ECV304 cells.
Free radicals play a significant role in the oxidation of LDL 28. In order to verify whether the oxidation is caused by free radicals, the level of free radicals at 2 and 10 h was tested. Considering that oxidative stress caused by free radicals could occur in a short time, we also tested the free radical levels at 1, 10, and 30 min. The results reveal that there are peaks at 2 and 10 h. In addition, HNP-1 has been shown to be involved in the oxidative stress in cells. Considering the simultaneous appearance of the peak values of HNP-1 and ROS, it is safe to hypothesize that the oxidation may be caused by free radicals.
As discussed above, the generation of MDA and HNP-1 mRNA fluctuates similar to that of the oxidation of LDL in our previous study. There may also be more than one oxidation pathway in this process. Thus, we performed the experiment to observe LDL oxidation in ECV304 induced by TFA at different times. The results reveal that the MDA in the medium of cells treated with LDL for 6 h followed by TFA treatment for 10 h is much higher than the MDA in the medium of cells treated with TFA for 16 h. In contrast, the cells treated with LDL for 6 h after being co-cultured with TFA for 2 h at the same concentration did not show a higher oxidation. In our previous research, we hypothesized that there may be two major ways of LDL oxidation by cells. Passive oxidation involves microbial infection and normal-level LDL oxidative damage. It is a process that the cells resist the stress induced by foreign matter. On the other hand, active oxidation involves high-level LDL oxidation damage 15. In the active oxidation group, MDA generation is much higher than it is in the passive group. It is a process that cells actively identify and remove the excessive substance in cells. In this study, we use TFAs as stimulants and there appear s two peak values in 24-h treatment with cells. Moreover, in the later peak, the oxidation seems to be more significant. We hypothesize that the former peak is a kind of passive oxidation and the later is a kind of active one. In order to explore the difference between the oxidation in the two time points, we use sodium ferulate, which is used as anti-radical drug, to further verify whether the oxidations were stimulated through radicals. According to the results, we found that MDA generation significantly decreases when sodium ferulate was added after cells are co-incubated with linoelaidic acid for 2 or 10 h. It is clear that in these two oxidation pathways, they are both stimulated by radicals.
To further explore whether MPO participates in the oxidation process, ABAH, a specific MPO inhibitor, combined with fresh LDL was added after cells were co-incubated with linoelaidic acid for 2 or 10 h. Through the results, we find in both groups, MPO activity is inhibited. However, MDA generation is only inhibited in the active oxidation pathway, but not in the passive oxidation pathway. In the group that verapamil and fresh LDL was added after cells are co-incubated with linoelaidic acid for 2 or 10 h, MPO activity is inhibited in both groups. However, MDA generation is inhibited in the passive oxidation pathway, but not in active oxidation pathway. It is widely known that calcium antagonists can reduce MPO activity and Ca2+-dependent signal pathway. It indicates that signal pathway may play a more important role in passive oxidation pathway.
Conclusions
In conclusion, all three TFA induce ECV304 cell apoptosis, and there is no significant difference among them. Oxidative stress can be induced by TFA in ECV304 cells. Furthermore, the oxidative stress can further stimulate the oxidation of LDL through the effects of free radicals. In the LDL oxidation process, there may be two kinds of oxidation pathways. In this case, the active oxidation pathway depends on the MPO activity and the passive oxidation pathway depends on the calcium ion signalling pathway.
Acknowledgements
This work was supported by grants from the Wuhan University (S2009354), Jingchu university of technology (ZR201008), National Natural Science Foundation of China (No. 30800400).
The authors have declared no conflict of interest.




