Effect of freezing on quality of sea bass and gilthead sea bream
Abstract
Freezing is an efficient method of fish preservation. The aim of the present work was to examine the impact of freezing in fatty acid composition and in the in vitro inhibitory activity of sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) fillet lipids against platelet activating factor (PAF). The Bligh and Dyer extraction method and the counter-current distribution method were used to obtain total, polar and neutral lipids. The fatty acid analysis conducted using the internal standard method and the biological assay on washed rabbit platelets took place calculating the in vitro inhibitory activity of fish lipids against 2.5 × 10−11 M of PAF. No statistical changes (p<0.05) occurred in fatty acid content of fresh and thawed gilthead sea bream, while fatty acid amount in thawed sea bass was significantly higher (p<0.05) compared to fresh fish. Total lipids of both thawed fish species exhibited stronger anti-thrombotic activity compared to fresh fish. Freezing preserved fish quality and reinforced the anti-thrombotic properties of fish oils, since even after 6 months of freezing, fish oils preserve their nutritional value in terms of protecting against cardiovascular diseases.
Practical applications: Fish fillets contain high amount of unsaturated lipids that may easily undergo lipid oxidation. Freezing and frozen storage prevent such oxidative changes so fish quality is retained. Fatty acids and PAF-antagonists in fish are of major importance since they contribute to the nutritional value of fish. The practical application of this work lies on the evaluation of the nutritional value of fish in terms of cardio protection by examining the impact of freezing on the levels of fatty acids and PAF-antagonists in aquacultured fish fillets.
Abbreviations:
EPA, eicosapentaenoic acid; NL, neutral lipids; PAF, platelet activating factor; PL, polar lipids; SFA, saturated fatty acids; TL, total lipids
Introduction
Freezing is an important method of fish preservation since frozen food can be stored for long period of time preventing microbial spoilage 1. Particularly fillet tissues undergo changes during freezing including formation of ice crystals and rupture of plasma membranes 2.
Upon thawing there is release of water and associated soluble components (drip loss from the fillet tissue) 1, 2. Factors influencing the nutrient stability of frozen foods include the freezing temperature of the unit and its range of fluctuation, length of storage, size of the cut, thawing method and packaging method 3.
Following the right freezing conditions fish sensory and nutritional properties can be retained; otherwise some deteriorative changes in fish fillet lipids may occur during freezing and long-term frozen storage, such as lipid oxidation and hydrolysis 4-7. Lipid peroxidation is caused by the high degree of unsaturated lipids, leading to radical production 8. These oxidative changes are mainly related to the taste and texture of the fish, while in later stages of lipid peroxidation, changes in colour and nutritional value can be observed 8. For this reason, freezing and frozen storage have largely been employed to retain fish sensory and nutritional properties 9.
Platelet activating factor (PAF) (1-O-alkyl-2-acetylsn-glyceryl-3-phosphocholine) 10 is a crucial inflammatory phospholipids mediator that is implicated in the mechanism of atherogenesis 11. Therefore, the presence of PAF-like molecules or PAF antagonists in fish and fish oil are very important in terms of health value.
Previous studies from our laboratory on lipids obtained from sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) 12, cod (Gadus morhua) 13, Scomber scombrus 14 and other fish species 15 have demonstrated the existence of compounds with anti-thrombotic properties that inhibit the actions of PAF. Moreover sea bass (D. labrax) and gilthead sea bream (S. aurata) lipid components with anti-PAF activity exhibited in vitro antibacterial properties 16 and managed to reduce in vivo the thickness of the atheromatic plaque in hypercholesterolemic rabbits 17. Our work is focused towards creating a novel fish with improved cardio protective activity by partially substituting fish oil in fish feed with natural components containing micro-constituents with beneficial properties for human health 18. In this context, the aim of the present research was to study the impact of freezing on fatty acid composition and on the in vitro PAF inhibitory activity of gilthead sea bream and sea bass fillet lipids.
Materials and methods
Chemicals
The solvents such as chloroform and all other solvents used were of analytical grade and supplied by Sigma (St. Louis, Mont, USA) and Merck (Darmstadt, Germany).
FAME standards bought individually were of GC-quality and supplied by Sigma (St. Louis, Mont, USA).
Fish fillet sampling and processing
Farmed gilthead sea bream (S. aurata) and sea bass (D. labrax) were obtained from marine farms, situated on Chios Island in May of 2006. Both species were fed with fish feeds of identical chemical composition. For each species, 36 fish were obtained and they were separated in twelve replicates, each replicate containing three fish. Fish specimens were beheaded, skinned, gutted and filleted and only the fillets were used in the subsequent experiments. The average weight of gilthead sea bream and sea bass fillets was 90 g. Six replicates of three fish each were the fresh fillet samples and were analyzed immediately while the rest six replicates of three fish each were packaged in polyethylene bags for freezing representing the frozen fillet samples. The frozen fillet samples were stored at −18°C for 6 months and then thawed [in a refrigerator (4 ± 1°C) overnight], minced (in a homogenizer) and analyzed as the fresh fish samples.
Isolation of fish fillet lipids
Total lipids (TL) of fresh and thawed fish fillets were obtained using the Bligh and Dyer method of lipid extraction 19. In brief, an appropriate amount of chloroform/methanol/water 1:2:0.8 v/v/v solution was added to each fish fillet sample of fresh (n = 6) or thawed (n = 6) fish and mixtures were shaken well and filtered (Whatman no. 1 filter paper was used). Phase separation of mixtures in the separatory funnels was achieved by adding chloroform and water to obtain a final chloroform/methanol/water ratio of 1/1/0.9 v/v/v. TL was obtained by the chloroform phase (lower phase) that was evaporated to dryness under nitrogen's stream and lipids were weighed and redissolved in 1 mL chloroform:methanol 1:1 v/v. One-tenth of the TL was stored under nitrogen in sealed vials at −20°C until used—after a short period of time—for the fatty acid analysis and the biological assay, while the rest of it was further separated into polar lipids (PL) and neutral lipids (NL) using the counter-current distribution method 20. In brief, this method is based on PL and NL different solubilities in pre-equilibrated petroleum ether and ethanol (87%). PL were soluble in ethanol while NL were soluble in petroleum ether. The obtained lipid fractions were weighed and stored under nitrogen in sealed vials at −20°C until used—after a short period of time—for the fatty acid analysis and the biological assay.
Fatty acid analysis
FAME of 35 mg fresh and thawed fish fillets TL, respectively, were prepared using 4 mL of 0.5 N KOH in CH3OH (KOH–CH3OH method) and extracted with 5 mL n-hexane. The reaction was completed after 5 min in RT. The fatty acid analysis was carried out using the internal standard method. A five point calibration curve was prepared using five solutions of heptadecanoic (17:0) acid methyl ester and heneicosanoic (21:0) acid methyl ester in ratios of 500:1000, 500:500, 500:200, 500:100 and 500:50 v/v, respectively.
Five injections of 1 µL of each solution were analyzed with a Shimadzu CLASS-VP (GC-17A) (Kyoto, Japan) gas chromatograph equipped with a split/splitless injector and flame ionization detector. The ratio of the mean area of (21:0) to that of the internal standard (17:0) is used as the y-axis variable of the calibration curve, while the concentration (mg/kg) of 21:0 is used as the x-axis variable of the calibration curve. The equation that described the calibration curve was y = 0.0012x + 0.0210, with r = 0.996. The ratio of the area of the analyte peak to that of the internal standard represents the y-value at the above equation and subsequently x-value represents the analyte concentration of the fatty acid in the unknown mixture.
Separation of FAME was achieved on an Agilent J&W DB-23 fused silica capillary column (60 m × 0.251 mm id, 0.25 µm; Agilent, Santa Clara California, USA). The oven temperature program was: initially 120°C for 5 min, raised to 180°C at 10°C/min, then to 220°C at 20°C/min and finally isothermal at 220°C for 30 min. The injector and detector temperatures were maintained at 220 and 225°C, respectively. The carrier gas was high purity helium with a linear flow rate of 1 mL/min and split ratio 1:50. FAME were identified using FAME standards (Sigma, St. Louis, Mont, USA) by comparison of the retention times of the relative peaks.
Biological assay on washed rabbit platelets
Total lipids of fresh and thawed fish fillets were studied for their in vitro PAF-inhibitory activity against 2.5 × 10−11 M PAF (final concentration) toward washed rabbit platelets. Washed rabbit platelets were prepared as described elsewhere 10.
Platelet activating factor-induced aggregation was measured as previously described 12. Platelet aggregation was measured in a Chrono-Log (Havertown, PA, USA) aggregometer (model 400-VS) coupled to a Chrono-Log recorder (Havertown, PA, USA) at 37°C with constant stirring at 1200 rpm. EC50 and IC50 values were calculated as previously described 12. EC50 accounts for the amount of each lipid fraction inducing aggregation equivalent to 50% PAF-induced aggregation and IC50 accounts for the amount of each lipid fraction inhibiting 50% PAF-induced aggregation.
Statistical analysis
All experiments were carried out six times and all results were expressed as mean ± SD. Normality tests were applied using the Kolmogorov–Smirnov criterion. Data were not normally distributed and non-parametric tests were performed. The Wilcoxon sign test was used to determine the significance of differences in the same group between fresh and thawed samples. Differences were considered to be statistically significant when p was less than 0.05. Data were analyzed using a statistical software package (PASW 18 for Windows, SPSS Inc.,Chicago, IL, USA).
Results and discussion
Total lipid, polar lipid, neutral lipid content
Total lipids, PL and NL in fresh and thawed sea bass and gilthead sea bream fillets expressed in g/kg are shown in Table 1.
| TL (g/kg) | NL (g/kg) | PL (g/kg) | |
|---|---|---|---|
| Fresh gilthead sea bream | 60 ± 6a | 24 ± 3a | 31 ± 3a |
| Thawed gilthead sea bream | 77 ± 7b | 32 ± 3b | 39 ± 4b |
| Fresh sea bass | 62 ± 7a | 26 ± 3a | 32 ± 5 |
| Thawed sea bass | 79 ± 8b | 36 ± 4b | 40 ± 5 |
- a, b in each column: indicates significantly different values between fresh and thawed gilthead sea bream and fresh and thawed sea bass, according to the Wilcoxon test (p<0.05).
Total lipids content in thawed fish of both species was significantly higher than TL content in fresh fish of both species (p<0.05). This is probably because during the thawing process a large quantity of water is lost because of the defrosting of ice crystals 1 and thus the fish fillet fat levels increase as the water content decreases 1, 21.
Neutral lipids and PL contribution to TL of fresh gilthead sea bream fillets was 40 and 52%, respectively, while NL and PL contribution to TL of thawed gilthead sea bream fillets was 42 and 51%, respectively. In fresh sea bass fillets, NL and PL contributed to TL 42 and 52%, respectively, while in thawed sea bass fillets NL and PL contribution to TL was almost equal (46 and 51%, respectively). This observation of almost equal contribution of NL and PL to TL of thawed fish is in accordance with literature 22.
Fatty acid content
Typical chromatograms of fresh and thawed sea bass and sea bream are shown in Figs. 1 and 2, respectively.
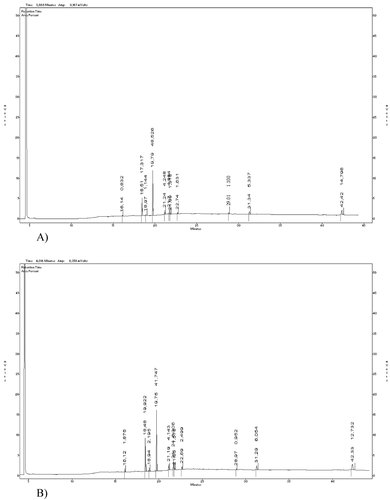
(A) Typical GC chromatogram of fresh sea bass TL (internal standard: heptadecanoic acid methyl ester (17:0); retention time 19.79 min). (B) Typical GC chromatogram of thawed sea bass TL (internal standard: heptadecanoic acid methyl ester (17:0); retention time 19.75 min).
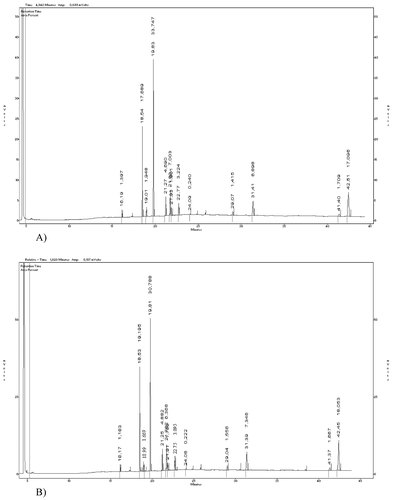
(A) Typical GC chromatogram of fresh gilthead sea bream TL (internal standard: heptadecanoic acid methyl ester (17:0); retention time 19.83 min). (B) Typical GC chromatogram of thawed gilthead sea bream TL (internal standard: heptadecanoic acid methyl ester (17:0); retention time 19.81 min).
The fatty acid composition (mg/kg) and changes during frozen storage in fresh and thawed sea bass and gilthead sea bream are summarized in Tables 2 and 3, respectively. Both species were fed with fish feeds of identical chemical composition.
| Fatty acids | Fresh gilthead sea bream (mg/kg) | Retention time (min) | Thawed gilthead sea bream (mg/kg) | Retention time (min) |
|---|---|---|---|---|
| 14:0 | 17.0 ± 3.40 | 16.19 | 14.0 ± 2.80 | 16.17 |
| 16:0 | 439 ± 20.9 | 18.54 | 489 ± 30.2 | 18.53 |
| 16:1 (ω-7) | 30.6 ± 4.82 | 19.01 | 26.8 ± 3.35 | 18.99 |
| 18:0 | 99.8 ± 7.12 | 21.27 | 109 ± 8.72 | 21.25 |
| 18:1 cis (ω-9) | 155 ± 9.73 | 21.80 | 153 ± 7.65 | 21.78 |
| 18:1 trans (ω-9) | 29.4 ± 2.08 | 21.93 | 30.3 ± 3.07 | 21.91 |
| 18:2 (ω-6) | 62.1 ± 4.14 | 22.77 | 65.7 ± 4.61 | 22.75 |
| 20:4 (ω-6) | 18.8 ± 1.77 | 29.07 | 23.0 ± 2.78 | 29.04 |
| 20:5 (ω-3) | 153 ± 11.9 | 31.41 | 178 ± 13.8 | 31.39 |
| 22:5 (ω-3) | 26.7 ± 2.33 | 41.40 | 23.5 ± 1.79 | 41.37 |
| 22:6 (ω-3) | 405 ± 19.9 | 42.51 | 446 ± 21.1 | 42.45 |
| Total SFA | 556 ± 23.9 | 612 ± 33.0 | ||
| Total MUFA | 215 ± 4.11 | 210 ± 4.09 | ||
| Total ω-3 PUFA | 585 ± 28.9 | 648 ± 35.8 | ||
| Total ω-6 PUFA | 80.9 ± 4.12 | 88.7 ± 5.71 | ||
| Total PUFA/SFA | 3.10 ± 0.42 | 3.51 ± 0.29 | ||
| Ω-3/ω-6 | 7.23 ± 1.02 | 7.31 ± 1.08 |
- SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; EPA, eicosapentaenoic acid.
- No significantly different values were found between fresh and thawed gilthead sea bream fatty acid composition, according to the Wilcoxon test (p>0.05).
| Fatty acids | Fresh sea bass (mg/kg) | Retention time (min) | Thawed sea bass (mg/kg) | Retention time (min) |
|---|---|---|---|---|
| 14:0 | 0.25 ± 0.05a | 16.14 | 20.2 ± 2.03b | 16.12 |
| 16:0 | 292 ± 23.5 | 18.51 | 344 ± 29.0 | 18.48 |
| 16:1 (ω-7) | 3.83 ± 0.77a | 18.97 | 30.2 ± 6.03b | 18.94 |
| 18:0 | 68.2 ± 3.12 | 21.24 | 70.6 ± 4.01 | 21.19 |
| 18:1 cis (ω-9) | 74.3 ± 4.19a | 21.76 | 119 ± 4.27b | 21.72 |
| 18:1 trans (ω-9) | 5.08 ± 1.02a | 21.89 | 14.2 ± 1.83b | 21.85 |
| 18:2 (ω-6) | 19.9 ± 2.58a | 22.74 | 33.8 ± 6.77b | 22.69 |
| 20:4 (ω-6) | 4.75 ± 0.55a | 29.01 | 6.33 ± 0.97b | 28.97 |
| 20:5 (ω-3) | 92.9 ± 8.62 | 31.34 | 109 ± 9.91 | 31.29 |
| 22:6 (ω-3) | 272 ± 24.4 | 42.42 | 249 ± 10.8 | 42.33 |
| Total SFA | 360 ± 19.1a | 435 ± 24.1b | ||
| Total MUFA | 83.2 ± 6.7a | 163 ± 11.6b | ||
| Total ω-3 PUFA | 365 ± 19.0 | 358 ± 16.7 | ||
| Total ω-6 PUFA | 24.7 ± 2.13a | 40.2 ± 6.03b | ||
| Total PUFA/SFA | 1.06 ± 0.16 | 0.92 ± 0.04 | ||
| Ω-3/ω-6 | 14.7 ± 3.25a | 8.91 ± 1.72b |
- SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; EPA, eicosapentaenoic acid.
- a, b in each row: indicates significantly different values between fresh and thawed sea bass according to the Wilcoxon test (p<0.05).
In fresh and thawed fillets of both species, palmitic (16:0), steatic (18:0) and oleic acids (18:1 ω-9) were the major fatty acids among the saturated and MUFA of both fish species (see Tables 2 and 3). It is noteworthy that both linoleic (18:2 ω-6) and AAs (AA, 20:4 ω-6) were predominant in the total n-6 PUFA (PUFAs) in fresh and thawed gilthead sea bream (see Table 2) while in fresh and thawed sea bass only linoleic acid (18:2 ω-6) was measured in high levels, since AA was only detected in small amounts (see Table 3). The decreased levels of AA in fresh and thawed sea bass in comparison to gilthead sea bream could be attributed to the relatively reduced amount of ω-6 PUFAs in sea bass compared to gilthead sea bream and probably due to different FA metabolism in the aforementioned fish species.
To date, there are rather limited data on the impact of freezing on the fatty acid profile of the two fish species studied here. To the best of our knowledge, the only study on the effect of freezing on fish components has focused on the levels of amino acids, rather than fatty acids, and took place on sea bass during frozen storage 23. In our study, eicosapentaenoic acid (EPA, 20:5 ω-3) and DHA (22:6 ω-3) account for most of ω-3 PUFA in fresh and thawed fillets of both fish species (see Tables 2 and 3). Analogous results were found for fresh gilthead sea bream (S. aurata) and sea bass (D. labrax) 24.
Distribution of fatty acids in fresh fillets of both species and in thawed gilthead sea bream was PUFA>saturated fatty acids (SFA)>MUFA (see Tables 2 and 3), whereas in thawed sea bass was SFA>PUFA>MUFA (see Table 3). The findings for the fatty acid distribution in fresh gilthead sea bream and sea bass are in agreement with the literature 25, 26. PUFA distribution in fresh and thawed gilthead sea bream is DHA>EPA>linoleic (18:2 ω-6)>docosapentaenoic acid (DPA 22:5) (see Table 2) and in fresh and thawed sea bass it is DHA>EPA>linoleic (18:2 ω-6) (see Table 3).
Regarding ω-3/ω-6 ratio it was suggested that a ratio of 1:1–1:4 would constitute a healthy human diet 27. In the current study, the content of ω-3 was found to be higher than the ω-6 compounds in fresh and frozen fish samples of both species (see Tables 2 and 3). Similar results have been obtained in mackerel (Scomberomorus commersoni) 28.
During frozen storage, no statistical changes were observed in fatty acid content in gilthead sea bream compared to thawed samples (see Table 2). On the other hand, in sea bass fillets, some fatty acids were significantly higher in thawed fish than in fresh fish. In particular, the SFA 14:0, the MUFA 16:1 ω-7, 18:1 ω-9 cis and 18:1 ω-9 trans and the ω-6 fatty acids 18:2 and 20:4 in thawed sea bass were significantly higher than in the fresh sea bass (see Table 3). Such alternations in fatty acid content of thawed sea bass fillets could be probably due to the loss of a large quantity of water during thawing process, resulting to increased fat and fatty acid levels 1, 21.
Biological properties of fish fillet lipids
The obtained TL, PL and NL fractions from fresh and thawed sea bass and gilthead sea bream were tested for their ability to induce washed rabbit platelet aggregation or inhibit the PAF-induced platelet aggregation. The EC50 and IC50 values of each lipid fraction from fresh and thawed gilthead sea bream and sea bass were expressed as mg of corresponding lipid fraction and are shown in Figs. 3 and 4, respectively.
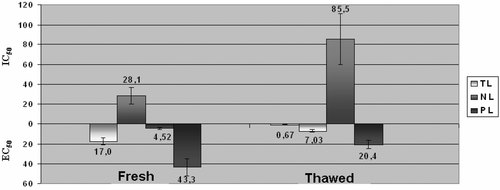
EC50 and IC50 values of TL, PL and NL of fresh and thawed gilthead sea bream expressed as mg of corresponding lipid fraction. All data are the average ± SD (95% confidence levels) of six replicate experiments. EC50 accounts for the amount of each lipid fraction (given here as actual mass in mg) inducing aggregation equivalent to 50% PAF-induced aggregation. IC50 accounts for the amount of each lipid fraction (given here as actual mass in mg) inhibiting 50% PAF-induced aggregation.
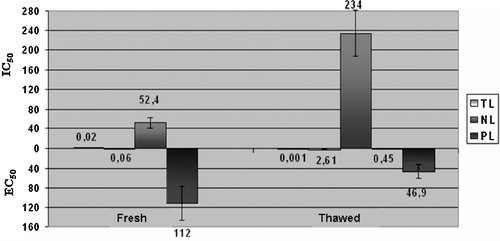
EC50 and IC50 values of TL, PL and NL of fresh and thawed sea bass expressed as mg of corresponding lipid fraction. All data are the average ± SD (95% confidence levels) of six replicate experiments. EC50 accounts for the amount of each lipid fraction (given here as actual mass in mg) inducing aggregation equivalent to 50% PAF-induced aggregation. IC50 accounts for the amount of each lipid fraction (given here as actual mass in mg) inhibiting 50% PAF-induced aggregation.
The TL of thawed gilthead sea bream and sea bass contained more potent PAF-agonists than the TL of the fresh fish species. This could be due to the reduction of water content and therefore to the increase of the composition of these biological active components in fish fillets after thawing. On the other hand NL of thawed gilthead sea bream and sea bass contained less potent PAF-inhibitors than the NL of the fresh fish species. Referring to PL, thawed gilthead sea bream exhibited less potent PAF-agonists than fresh gilthead sea bream, while thawed sea bass exhibited more potent PAF-agonists than fresh sea bass.
The presence of molecules with platelet aggregating properties (PAF-agonists) in TL and PL of both fish species does not necessarily mean that these molecules have prothrombotic effect since they are less active than PAF. The presence of such molecules in the bloodstream and their binding to PAF receptors could minimize the biological actions of PAF and in other words they could be considered as PAF-inhibitors.
Conclusions
In conclusion, TL content in thawed fish of both species increased in comparison with TL content of fresh fish, given the fact that during the thawing process, a large quantity of water was lost. During frozen storage, no statistical changes were observed in fatty acid content in gilthead sea bream comparing to thawed samples, while in sea bass fillets, some fatty acids were significantly higher in thawed fish than in fresh fish probably due to the loss of a large quantity of water during thawing process resulting to increased fish fillet fat levels and thus increased fatty acid amounts. In addition the ω-3 fatty acids content was larger than the ω-6 compounds both in fresh and frozen fish samples.
The biological assay showed that TL of both thawed fish species possessed more potent anti-thrombotic activity in comparison with fresh fish.
The overall conclusion is that freezing is a good preservation method for fish, preventing spoilage, extending product shelf life and according to the present study reinforces the anti-thrombotic properties of fish, since even after 6 months of freezing, fish preserves its nutritional value in terms of protecting against cardiovascular diseases.
The authors have declared no conflict of interest.




