Antifungal and antibacterial effects of 1-monocaprylin on textile materials
Abstract
The efficacy of a new antimicrobial treatment of textile materials based on the use of 1-monocaprylin against various species of saprophytic moulds (Alternaria alternate CCM F-397, Aspergillus niger ATCC 16404, Mucor racemosus CCM 8190, Penicillium ochrochloron CCM F-158, Trichoderma viridae CCM F-728), pathogenic moulds (Epidermophyton floccosum CCM 8339, Trichophyton mentagrophytes ATCC 9533, Trichophyton rubrum DSMZ 4167), pathogenic yeasts (Candida albicans ATCC 10231, Candida parapsilosis CCM 8260), Gram-negative bacteria (Escherichia coli ATCC 11229, Klebsiella pneumoniae ATCC 4352) and Gram-positive bacteria (Staphylococcus aureus ATCC 6853) was investigated. The testing was carried out according to DIN EN ISO 20645 disc-diffusions test, using a 2-layer method. The results showed that 1-monocaprylin effectively killed all tested bacterial strains and pathogenic microorganisms with an exception of saprophytic moulds only, which were partially resistant. Textiles treated with 1-monocaprylin reduced the growth of pathogenic, potentially dangerous microorganisms frequently occurring for example on the feet and in the shoes.
Practical applications: To prevent microbial contamination leading to degradation of textile materials, various antimicrobial agents aimed at killing or suppressing of microorganism growth are applied. Among others, also MAGs belong to safe and efficient antimicrobial agents. Their application in antimicrobial treatment of textiles may be a suitable alternative to commercially used antimicrobial agents, as these endogenous lipid substances are present almost in all animal and plant tissues and are harmless to human body. The present study has confirmed that MAGs possess a suitable inhibitory activity when applied on textiles and are capable of hindering and even suppressing growth of bacteria and moulds that may occur during storage and use of textiles. The obtained results can be closely related to potential industrial applications of MAGs as effective agents for antimicrobial textiles and lining and insole materials for footwear, including those designed for diabetics.
Abbreviations:
CCM, Czech collection of microorganisms; FA, fatty acid; PA, polyamide; PES, polyester
Introduction
Textile products from natural and synthetic fibres come into contact not only with the skin but also with the weather conditions. Moreover, they are also exposed to climatic factors during the storage indoors and influenced by the presence of nutrients, which play an important role as they directly influence the microbial growth on the textiles 1.
Natural fibres based on cellulose (cotton, tow, jute) and proteins (wool, silk) can easily become a culture medium for microorganisms capable of cleaving the fibre macromolecules into simple sugars and amino acids, respectively 2. Synthetic fibres, due to their hydrophobicity, are more resistant to attacks by microorganisms than natural fibres 3 and polyamide (PA), polyester (PES), polyacrylonitrile (PAN) and polypropylene (PP) are the basic types of synthetic materials used in the textile industry 1, 2.
The destruction of textile fibres is caused by enzymes produced by microorganisms and is manifested through the deterioration of physico-mechanical properties of the attacked materials. According to their nature, microbial enzymes can cleave the cellulose to oligosaccharides and simple sugars that are carbon and energy sources for the microorganisms. More than 70 types of such bacteria and fungi have already been isolated. The most important fungi genera destructing the cellulose fibres are Aspergillus, Penicillium and Trichoderma 1.
This situation is similar for proteins in keratinous fibres and carbohydrates in cotton both acting as nutrients together with soil, dust, solutes from sweat and certain textiles finishes 3.
To avoid the destruction of the textiles by microorganisms, the following ways can be used: (i) a modification of textiles by the fixation of substances with a lethal effect on microorganisms; (ii) a coating of the textile fibres surface with the film containing the substance that is inert against microorganisms activity; (iii) a change of the fibres surface to obtain derivatives that are not attacked by microorganisms 4.
Prevention the microbial contamination followed by degradation of textiles can be achieved through different antimicrobial treatments aimed at killing or suppressing growth and reproduction of bacteria, fungi and yeasts 5, 6.
The basic requirements for antimicrobial agents applied on textiles have been briefly reviewed by Purwar and Joshi 3 and Williams et al. 7 and include safety, high adhesion to the fibres, good emulsifying properties in water or ethanol and absence of interference with other material properties of treated textiles 2, 8, 9.
Naturally, these substances must exhibit sufficient antimicrobial activity with a wide spectrum of action against bacteria, yeast and fungi, low toxicity and good compatibility with the skin. An ideal antimicrobial substance should remain stable, even after prolonged application time, and should not be influenced by external factors (light, oxygen, heat, humidity, etc.) 2.
The use of natural products such as chitosan and natural dyes for antimicrobial finishing of textiles has been widely reported. Other natural herbal products including Aloe vera, tea tree oil, Eucalyptus oil and tulsi leaf (Ocimum basilicum) extracts, can also be used for this purpose 10-12. Also MAGs 13 and fatty acids (FAs) are examples of antimicrobial agents that can be used for the textile protection 14. From the safety point of view, application of the latter two substances seems to be particularly interesting, as they are both commonly used in food industry and have GRAS status (Generally Recognized as Safe) in the United States and within the EU 15. Research interest is mainly focused on MAGs based on saturated FAs with an even carbon number in the chain, such as 1-monocaprylin (MAG 8:0), 1-monocaprin (MAG 10:0) and 1-monolaurin (MAG 12:0) 16-20.
Monolaurin is the most frequently studied MAG and it was proved that even a lauric acid has a certain degree of ability to suppress the growth of microorganisms 18, 21-23. Here, some examples are presented. Inhibitory effects of monolaurin in combination with lactoperoxidase, thiocyanate and H2O2 were confirmed by Mc Lay et al. 24. The study of Kabara 20 demonstrated that 1-monolaurin exhibits not only increased antimicrobial but also emulsifying efficiency, which offers a possibility for the applications in foods. Neyts et al. 17 studied hydrogels containing 1-monocaprin and they also demonstrated a significant microbicidal activity against skin and sexually transmitted infections. MAGs were also tested as antimicrobial textile finishing. The inventors of US patent 25 performed treatment of non-wovens with MAG 12:0 in combination with chitosan, lauric acid and castor oil ethoxylates/PEG diesters, respectively. During the treatment, polyolefine and PES non-woven fabrics were sprayed with aqueous emulsions of the tested substances and antimicrobial efficacy against Staphylococcus aureus and Klebsiella pneumoniae was tested. It was found out that only combination of MAG 12:0 and chitosan is efficient against both the tested strains 25.
Microbiological research suggests that the bactericidal effects of higher FAs and MAGs are related to properties of bacterial cell wall. It was shown that Gram-positive bacteria are more sensitive to antimicrobial agents than Gram-negative ones 26, 27. The reason for the increased resistance of Gram-negative bacteria is composition of their outer cell membrane, which is richer in lipopolysaccharides forming a protective layer on the bacterial surface 28.
The aim of this study was hence to elucidate the antimicrobial activity of 1-monocaprylin (MAG 8:0), applied to the textile materials, against saprophytic fungi, pathogenic fungi and yeasts, as well as against Gram-positive and Gram-negative bacteria.
Materials and methods
Monoacylglycerol and textile materials
1-Monocaprylin was prepared by the reaction of caprylic acid (Sigma–Aldrich, St. Louis, MO, USA) with glycidol (Sigma–Aldrich) in the presence of chromium acetate hydroxide (Sigma–Aldrich) as a catalyst 29. Prepared monoacylglycerol (MAG C8:0) was purified using double recrystallization from ethanol (Sigma–Aldrich). The purity of MAG was higher than 99%.
The following textiles were used in the test: cotton, PA, PES, commercial two-layer lining material Isofix (PA with polyurethane) and Isofix treated with antimicrobial finish Sanitized GT88-06 (Sanitized AG, Switzerland), all obtained from the company Tomatex Otrokovice, a.s., CZ. Basic characteristics of textile materials are given in Table 1.
| Material | Fibre thickness (mm) | Weight (g/m2) | Density (g/cm3) | Structure of fabric | Density of warp | Density of weft |
|---|---|---|---|---|---|---|
| Polyamide | 0.41 | 120 | – | Non-woven | n.a. | n.a. |
| Polyester | 0.77 | 190 | – | Knitted | – | – |
| Cotton | 0.65 | 180 | – | Fabric | 350/10 cm | 190/10 cm |
| Isofix | 0.85 | 150 | 0.18 | Non-woven | n.a. | n.a. |
- n.a., not applicable. Remaining data are not provided by the manufacturer.
Tested microorganisms and culture media
Test cultures of Aspergillus niger ATCC 16404, Trichophyton mentagrophytes ATCC 9533, Candida albicans ATCC 10231, Escherichia coli ATCC 11229, Klebsiella pneumoniae ATCC 4352 and Staphylococcus aureus ATCC 6853 were obtained from American Collections of Microorganisms. Alternaria alternata Czech collection of microorganisms (CCM) F-397, Trichoderma viridae CCM F-728, Penicillium ochrochloron CCM F-158, Mucor racemosus CCM 8190, Epidermophyton floccosum CCM 8339, Candida parapsilosis CCM 8260 were supplied from the CCM. Culture of Trichophyton rubrum DSMZ 4167 was received from the German Collection of Microorganisms and Cell Cultures in Braunschweig. The Czapek Dox Agar (Imuna, Slovakia) was used for cultivation of saprophytic fungi (Alternaria alternata, Trichoderma viridae, Aspergillus niger, Penicillium ochrochloron, Mucor racemosus), Sabouraud 2% Glucose Agar (Sigma–Aldrich) for the cultivation of yeasts (Candida albicans, Candida parapsilosis) and pathogenic fungi (Trichophyton mentagrophytes, Trichophyton rubrum, Epidermophyton floccosum) and Tryptone Glucose Extract Agar (HiMedia Bombai, India) for the cultivation of bacteria (Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus). Selection of microorganisms was motivated as follows: Staphylococcus aureus, Klebsiella pneumonia and Escherichia coli are generally recommended for antimicrobial tests in most of the test methods 30, 31 mainly due to the fact that the former two species are potentially pathogenic for humans. Alternaria, Aspergillus, Penicillium and Trichoderma are the common fungi genera destructing the cellulose fibres and genera Trichophyton, Epidermophyton and Candida are pathogenic for humans.
Determination of effective MAG concentration
The effective concentration of MAGs for textile treatment was determined by disc-diffusion test DIN EN ISO 20645 32 using a 2-layer method. A standard concentration of bacterial cell suspension of 107–108 CFU/mL was applied. The Czapek Dox Agar was used for cultivation of saprophytic fungi (Aspergillus niger), Sabouraud 2% Glucose Agar for cultivation of yeasts (Candida albicans) and pathogenic fungi (Trichophyton mentagrophytes) and Tryptone Glucose Extract Agar for cultivation of bacteria (Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus).
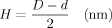 ((1))
((1))On the basis of these disc-diffusion tests the most effective concentration of MAG 8:0 was selected and subsequently used for modification of textile materials.
Preparation and modification of textiles
From the tested textile materials (cotton, PA, PES, Isofix), the round-shaped test specimens with a diameter of 30 mm were cut and sterilized under UV light for 40 min from both sides. Subsequently, they were immersed in the ethanolic MAG 8:0 solution of the optimal concentration determined in the previous test and placed on a shaker for 6 h at the temperature of 25°C. The samples were then dried in a laboratory oven at 35°C for 1 h in order to evaporate ethanol. Prior to antimicrobial testing, each side of MAG modified textiles was sterilized under UV light for 40 min again. The amount of bounded MAG 8:0 was determined, with an accuracy of 0.0001 g, from the weight differences of textile samples before and after modification and expressed as µg of MAG per g of textile material.
Antimicrobial testing of modified textiles
Though a number of test methods have been developed to determine the efficacy of antimicrobial textiles 30, 31 testing of modified textile materials was carried out by the disc-diffusion test DIN EN ISO 20645 32 using 2-layer method. The spectrum of microorganisms used in this test was wider than that applied in the effective concentration determination. A standard concentration of bacterial cell suspension was of 107–108 CFU/mL. Both upper and bottom sides of each textile material were tested and all experiments were done in duplicates. Cultivation was performed at 25 ± 1°C (yeasts, pathogenic fungi and saprophytes) and at 37 ± 1°C (bacteria). The microbial growth was monitored after 1, 2, 7, 14 and 30 days of cultivation (fungi and yeasts) and after 1, 2 and 5 days of cultivation (bacteria).
Antimicrobial efficacy of modified textiles was expressed as the presence or absence of inhibition zone and the growth of microorganisms below and on the surface of the sample.
Results and discussion
Determination of effective MAG concentration
Based on the disc-diffusion tests with filters modified by ethanolic MAG 8:0 solutions, the inhibition zone sizes and antimicrobial effects of various MAG C8:0 concentrations were evaluated. As it can be seen in Fig. 1, the inhibition zone sizes increased with higher MAG concentrations. From the figure it is obvious that 15 wt% MAG 8:0 solution in ethanol showed comparable antimicrobial effects as 20 wt% solution. However, MAG concentration of 15 wt% was chosen as the optimum concentration for further modification of textile materials. The reason for the selection of this concentration was motivated by similarity of antimicrobial effects of both concentrations and easier preparation procedure for 15 wt% MAG solution, which did not require heating. Moreover, visual inspection of treated textiles did not reveal any changes of the surface of tested textiles in terms of colour, structure and character. Thus, all the textiles under test (PA, PES, cotton, Isofix) were modified by immersing to15% MAG solution.
Effective concentration of MAG 8:0 for textile modification determined from inhibition zone sizes (H) (concentrations of 2–6 wt% are not shown).
The amount of MAG 8:0 absorbed on textile materials
The amount of MAG absorbed on textile materials was determined and expressed as µg per g textile material (Table 2). It is evident that each material shows a different ability to absorb the active substance into its structure, which is dependent on the type of fibres, their thickness, weaving density, etc. The highest amount of MAG was absorbed by Isofix. This is mainly due to the structure of material that is composed of two layers, one of which is foam polyurethane. This porous foam can soak the MAG solution into its structure increasing thus the total amount of absorbed MAG. It is however possible to suggest that MAG absorbed inside textile do not increase antimicrobial effect of MAG, which is mainly governed by MAG present on the surface.
| Type of material | Type of modification | Amount of absorbed MAG per weight of textile material (µg/g) |
|---|---|---|
| Polyamide | MAG C8:0 (15 wt% solution) | 315.90 ± 0.10 |
| Polyester | 175.80 ± 0.06 | |
| Cotton | 152.30 ± 0.15 | |
| Isofix | 606.10 ± 0.06 |
Antifungal activity of MAG 8:0 against saprophytic fungi
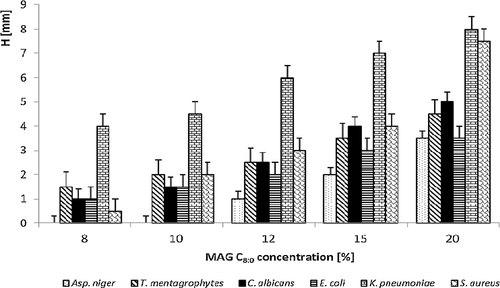
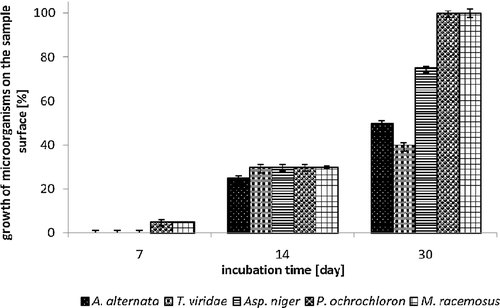
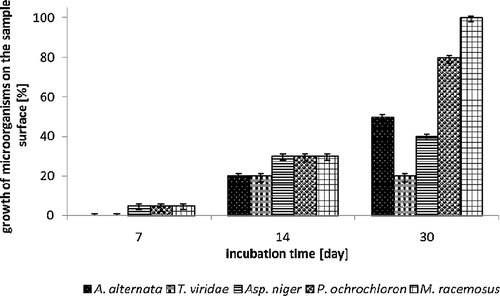
The first read-out of antifungal effect of PA, PES, cotton textiles modified with 15 wt% MAG 8:0 ethanolic solution was carried out after 24 h. The results showed no growth of any of the tested saprophytic fungi, even in any tested material; moreover, in all cases, presence of inhibition zone was observed. Correspondingly, the second day of cultivation, an inhibition zone was observed again for all tested textile materials. The seventh day of cultivation, a growth of cultures Aspergillus niger, Penicillium ochrochloron and Mucor racemosus occurred on samples of PES and cotton, covering ∼5% of the material surface. The growth on the agar surface, under the textile samples was not observed. Evidently, PA textile (Fig. 2) proved to be the most resistant material; no growth (neither on the surface nor under the sample) of Aspergillus niger was observed. All three tested textiles were also reasonably resistant to Alternaria alternata with only a partial growth which appeared after 14 days of testing, covering of about 20–30% sample surface. While the growth of all tested saprophytic fungi was recorded on the sample surface, no growth was observed under the sample surface. Thirtieth day of testing, the samples surfaces were mainly overgrown, which was caused by the growing hyphae of filamentous fungi and aerobic nature of their metabolism. In all tested textile materials the decreasing of their growth rate was visible (Figs. 2, 3 and 4). Though MAG 8:0 modified materials showed an antifungal effect, it was not strong enough to suppress growth of fungi throughout the whole cultivation period. Antifungal activity of MAG 8:0, MAG C10:0 and MAG 12:0 against filamentous fungi (Aspergillus niger, Penicillium roqueforti, Penicillium jensenii, Alternaria sp., Phoma sp.) was tested by Buňková et al. 33 who reported that all the tested MAGs exhibited antifungal efficacy at the concentrations equal or higher than 32.5 mg/L. Comparison of the three MAGs proved that monolaurin showed better inhibition of Monascus ruber. However, the growth of the less resistant Penicillium species was better inhibited with MAG 8:0, MAG 10:0.
Inhibition effect of MAG 8:0 on PA textile expressed as growth of microorganisms on the agar plate (%) depending on incubation time. Used fungi: A. alternata, T. viridae, Asp. niger, P. ochrochloron, M. racemosus.
Inhibition effect of MAG 8:0 on PES textile expressed as growth of microorganisms on the agar plate depending on incubation time. Used fungi: A. alternata, T. viridae, Asp. niger, P. ochrochloron, M. racemosus.
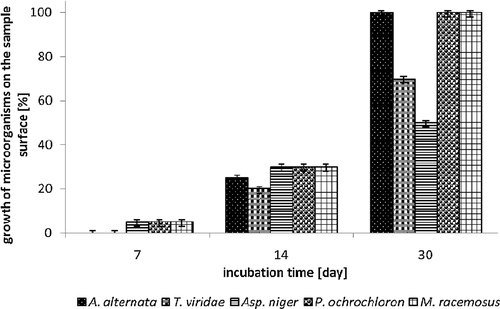
Inhibition effect of MAG 8:0 on cotton textile expressed as growth of microorganisms on the agar plate depending on incubation time. Used fungi: A. alternata, T. viridae, Asp. niger, P. ochrochloron, M. racemosus.
Antifungal activity of MAG 8:0 against pathogenic fungi
Saprophytic fungi may represent a source of possible destruction of textile fibres. However, from the health-hazard viewpoint, they are not as dangerous species as the pathogenic fungi are. Therefore, the antifungal effect of textiles modified with 15 wt% solution of MAG 8:0 was mainly tested against representatives of pathogenic fungi, specifically against Trichophyton mentagrophytes, Trichophyton rubrum and Epidermophyton floccosum. First 7 days of testing, the MAG modified materials showed very satisfactory antifungal effects with ‘no growth’ observed. The inhibition zone was observed up to 14th day of cultivation, when the growth of Trichophyton mentagrophytes (app. 5%) appeared on the PA sample surface that continued further days of testing. The antifungal effect of modified PA samples against Epidermophyton floccosum was evident even after 30 day of testing, the presence of inhibition zone was still observed. For PA samples, the growth of Trichophyton mentagrophytes and Trichophyton rubrum was recorded on the 14th and 30th day of testing, respectively. For both cultures growth on the surface of the textiles was observed covering ∼5% of their surfaces.
PES and cotton also showed a very satisfactory antifungal activity, since no growth of pathogenic microorganisms was observed even after 30 days of testing neither on the culture medium under the sample nor on the sample surface. Inhibition zone was visible during the entire period of testing.
Antimicrobial activity of MAG 8:0 against pathogenic yeast
Pathogenic yeasts of the Candida genus (Candida albicans and Candida parapsilosis) were selected for this test. Also in this case, the treatment with MAG 8:0 was effective for inhibition of yeast growth throughout the whole investigated period; the presence of inhibition zones was visible even after 30 days of testing. Testing of MAG 10:0 against fungal microorganisms was carried out by Kabara 20 with the major representatives of yeast (Candida albicans, Saccharomyces cerevisiae) and filamentous fungi (Aspergillus and Penicillium). Also here, MAG acted as an efficient agent and for Candida albicans, minimum inhibitory concentration (MIC) was determined at 200 mg/L.
Antimicrobial activity of MAG 8:0 against Gram-positive and Gram-negative bacteria
To prove or disprove the complexity of the antimicrobial effect of MAG C8:0, the modified textile materials were tested against both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli, Klebsiella pneumoniae) bacteria.
The results showed that 1-monocaprylin was able to suppress the growth of all tested bacteria. The presence of inhibition zone for all observed materials (PA, PES, cotton) was recorded even after 30 days of testing. Analogous conclusions, showing suppression of bacterial growth by MAG 10:0 and MAG 12:0, were published by Růžička et al. 34. Surprisingly, MAG 10:0 was more efficient of both tested substances and was found to inhibit the growth of all tested Gram-positive and two cultures of Gram-negative bacteria, Klebsiella pneumoniae and Acinetobacter lwoffii. Cultures of Gram-negative Escherichia coli and Pseudomonas aeruginosa were, however, resistant. MAG 12:0 was more efficient against Bacillus subtilis, Staphylococcus aureus and against Acinetobacter lwoffii, whilst no inhibitory effect was observed against other Gram-negative bacteria.
Comparison of antimicrobial effect of MAG 8:0 with a commercial antimicrobial agent SANITIZED GT88-06
The textile material Isofix, which is used as a vamp lining material of diabetic footwear, was chosen in order to compare antimicrobial activity of MAG 8:0 and commercially available antimicrobial agent SANITIZED GT88-06. Isofix material was provided both untreated and treated with SANITIZED GT88-06. Untreated Isofix was then modified with 15 wt% solution of MAG 8:0 in ethanol. For comparison of antimicrobial effects, representatives of microorganisms, which showed the maximum viability in the previous tests (Aspergillus niger ATCC 16404, Trichophyton rubrum DSMZ 4167, Candida albicans ATCC 10231, Escherichia coli ATCC 11229, Klebsiella pneumoniae ATCC 4352 and Staphylococcus aureus ATCC 6853), were used.
Surprisingly, commercially available material Isofix with antimicrobial modification SANITIZED GT88-06 did not show any antibacterial effect. After the first read-out (24 h) Isofix was resistant only against Staphylococcus aureus and did not provide any protection against tested bacteria after 2 days of incubation (Table 3).
| Incubation time (days) | 1 | 2 | 5 | 7 | 14 | 30 | |
|---|---|---|---|---|---|---|---|
| Tested microorganisms | Growth of microorganisms below the sample on the agar plate (% of area) | ||||||
| Isofix with SANITIZED GT88-06 | E. coli | 80.0 ± 6.1 | 83.8 ± 4.1 | 100.0 ± 0.0 | N | N | N |
| K. pneumoniae | 58.8 ± 4.1 | 70.0 ± 3.5 | 100.0 ± 0.0 | N | N | N | |
| S. aureus | 0.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | N | N | N | |
| Asp. niger | 0.0 ± 0.0 | 0.0 ± 0.0 | N | 0.0 ± 0.0 | 32.5 ± 2.5 | 56.3 ± 2.2 | |
| T. rubrum | 0.0 ± 0.0 | 0.0 ± 0.0 | N | 10.0 ± 0.0 | 60.0 ± 3.5 | 85.0 ± 3.5 | |
| C. albicans | 0.0 ± 0.0 | 0.0 ± 0.0 | N | 22.5 ± 2.5 | 32.5 ± 2.5 | 58.8 ± 2.2 | |
| Isofix with MAG 8:0 | E. coli | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | N | N | N |
| K. pneumoniae | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | N | N | N | |
| S. aureus | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | N | N | N | |
| Asp. niger | 0.0 ± 0.0 | 0.0 ± 0.0 | N | 0.0 ± 0.0 | 5.0 ± 0.0 | 56.7 ± 2.4 | |
| T. rubrum | 0.0 ± 0.0 | 0.0 ± 0.0 | N | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.0 ± 1.4 | |
| C. albicans | 0.0 ± 0.0 | 0.0 ± 0.0 | N | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
- N – not tested.
On the contrary, Isofix material modified by 15% solution of 1-monocaprylin showed an excellent antibacterial activity against all test bacterial cultures. During the whole testing period, the presence of inhibition zones has been observed.
Furthermore, both materials were subjected to testing against yeasts and fungi including pathogens. The tests revealed that antifungal activity of Isofix with a commercial finish SANITIZED GT88-06 was significantly better than the antibacterial one. Resistance against Aspergillus niger, Trichophyton rubrum and Candida albicans was disrupted on the seventh day of testing (Table 3) when the growth of pathogenic yeasts Candida albicans (∼20% of the area) and pathogenic fungi Trichophyton rubrum (∼10% of the area) was detected under the sample surfaces. In addition, the 14th day of testing, the growth of saprophytic fungi Aspergillus niger occurred on the sample surface and the fungi covered the sample surface area of about 30%. Within the further testing period, the area covered by microorganisms increased. It can be concluded, that the lining material with the antimicrobial finish Isofix SANITIZED GT88-06 shows only a time limited antifungal activity.
On the other hand, the antifungal activity of the Isofix modified with MAG 8:0 was disrupted by the growth of saprophyte Aspergillus niger first on the 14th day of testing, when the microbial growth under the sample was observed. High antifungal efficacy of MAG modified material was proved against fungi Trichophyton rubrum, since the growth under the sample was observed on the 30th day of testing. In case of pathogenic yeast Candida albicans, no growth was showed and the presence of inhibition zone was observed for 30 days of testing (Table 3).
The satisfactory antifungal activity and the absence of antibacterial effect of Isofix modified with SANITIZED give evidence that the commercial modification is used primarily to protect materials against yeasts and fungi than against bacteria. Moreover, the antifungal modification with the SANITIZED GT88-06 is not long-lasting; as its effectiveness was demonstrated only for 7 days. The modification with MAG 8:0 provides on the other hand sufficient antimicrobial protection of Isofix and, in contrast to the commercial product, warrants significantly longer antimicrobial activity against all pathogenic (14 days) and saprophytic microorganisms (7 days).
Simultaneously, the blank experiments on materials without any antimicrobial treatment were carried out in the same way. The comparison of treated and non-treated textiles verified that MAG is the only active substance assuring the antimicrobial effect (Fig. 5).
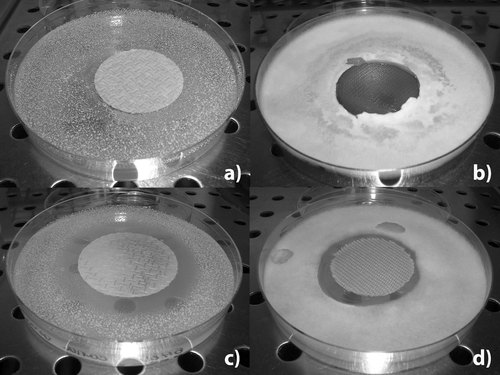
Inhibition effect of MAG 8:0 on PA and PES textiles against Candida albicans and Trichoderma viridae after 7 days. (a) PA textile without treatment (b) PES textile without treatment (c) PA textile treated with MAG 8:0 (d) PES textile treated with MAG 8:0.
Conclusions
It has been experimentally demonstrated that PA, PES, cotton and ISOFIX textile materials modified with 15 wt% MAG 8:0 solution strictly suppressed the growth of pathogenic yeast (Candida albicans and Candida parapsilosis), as well as Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli, Klebsiella pneumoniae) bacteria. To some extent, the MAG modified textiles prevented also the growth of pathogenic fungi and slowed growth of saprophytic fungi. The obtained results indicate that the microbial growth at the tested concentration of MAG is dependent on the type (cellulose, PA, etc.) and structure of fibres (non-woven, knitted, fabric). The highest microbial growth was observed for the cotton samples, serving as a possible source of nutrients for microorganisms.
The treatment of textile materials with MAG 8:0 provided sufficient antimicrobial protection against wide spectrum of microorganisms and MAG 8:0 can be, hence, applied as an effective antimicrobial agent for commonly used textiles, whether industrial, as well as the lining and insole materials for footwear, including those designed for diabetics. The MAG ethanolic solution can be applied on textile materials by spaying or it can be used for antimicrobial protection of disposable products. Hence, this treatment can be with advantage use for vamp lining and insole materials which are not expected to be washed.
The obtained results confirm the possibility for further potential use of 1-monoacaprylin for antimicrobial treatment of textiles with regard to its effectiveness, biocompatibility and biodegradability.
Acknowledgements
Authors thank to Assoc. Prof. Růžička, Assoc. Prof. Hlaváček and Dr. Sedlaříková for kind assistance. The work was supported by a project of the Ministry of Education, Youth and Sports of the Czech Republic No. MSMT 7088352101.
The authors have declared no conflict of interest.




