Glycolipids improve lutein bioavailability and accumulation in eyes in mice
Abstract
Intestinal carotenoid absorption is greatly affected by dietary factors. In this study, it was hypothesized that lipids with varying functional groups may influence differentially lutein bioavailability. Hence, the influence of glyco-, phospho-, neutral, crude (mixture of lipids) lipids, or mixed micelles (control) on the percent lutein micellarization in vitro and its postprandial plasma, liver, and eye response in mice were investigated. Results show that the percent micellarization of lutein with crude lipids and glycolipids were higher (91.4 and 45.7%) than control, while no significant difference was found between phospho- and neutral lipids. The mean plasma response of lutein was higher for crude- (6 times), glyco- (3 times), phospho- (2.7 times), and neutral (2 times) lipid than control (12.4 ± 1.18 nmol/mL 8 h−1) group. Lutein levels (pmol/g) in liver were higher in crude (7.4 ± 1) and phospho- (3.6 ± 0.8) lipid groups while in eyes it was higher in glyco- (54.0) and neutral (21.2) lipid groups than control. The influential effect of glyco- and phospholipids may be due to smaller micellar size (glyco-upto 3.43 µm, phospho- upto 5.78 µm) than the neutral lipids (upto 66 µm). Ingestion of lutein with glycolipid or phospholipids may improve lutein bioavailability.
Practical applications: The findings of the present study will be useful in nutritional and biomedical applications for feeding lutein with specific lipid combinations to achieve enhanced lutein absorption. Specifically, feeding diet/emulsion with lutein and glyco- and phospholipid combination may reduce the risk of macular degeneration, owing to the influential effect of these lipids on intestinal absorption of lutein.
Abbreviations:
AMD, age-related macular degeneration; AUC, area under the curve; OCC, open column chromatography
Introduction
Age-related macular degeneration (AMD) is one of the leading causes of blindness in the elderly. The incidence of AMD is expected to increase and may reach close to three million in another 20 years 1. Lutein, one of the natural carotenoid belonging to xanthophyll family and its isomer zeaxanthin are highly concentrated in the macula lutea of human eyes and could contribute in reducing AMD by virtue of their antioxidative property 2. They can quench singlet oxygen and other reactive oxygen intermediates 3. Studies show that lower lutein levels in eyes are at risk of developing AMD 4, 5. Lutein is an important antioxidant carotenoid for macula and human cannot synthesize it. Dietary ingestion is the only source to meet its requirements. However, lutein being a lipid soluble pigment, dietary lipids can probably influence its absorption rate, which limits its bioavailability.
Many studies focused on the effect of dietary factors including lipid on intestinal absorption 6, 7, but very few studies are available on the role of specific lipids on their bioavailability 8. Intestinal absorption of lutein depends on the concentration and origin of dietary fat consumed 9, 6. Garrett et al. 7 studied the role of high and low fat on β-carotene absorption and reported improved plasma lutein response after a meal with sufficient fat, but reduced when fat is absent or too low. Baskaran et al. 8 and others 10 studied the effect of phospholipid micelles on the β-carotene and lutein uptake in rodents and human intestinal Caco-2 cells, respectively, demonstrating importance of specific lipids on carotenoid absorption.
Glyco-, phospho-, and neutral lipids vary with their chemical structure, fatty acid profile and may have disparity on lutein absorption, being different in polarity. Sugawara et al. 10 and Lakshminarayana et al. 6 studied the effect of phospholipids on β-carotene and lutein absorption, respectively and reported that phosphatidylcholine with long chain acyl moieties suppress whereas long-chain lyso-phosphatidylcholine enhance their uptake from mixed micelles by Caco-2 cells and rats. These studies strongly suggest that carotenoid uptake is dependent on the nature of lipids (polar head groups and fatty acid profile) in which carotenoids are solubilized.
In this study, we have hypothesized that lipids with diverse functional (polar) groups may have differential effect on the lutein bioavailability. Hence, the present study was aimed to investigate the effect of glyco-, phospho-, neutral, and crude lipids on the plasma, liver, and eye response of lutein and plasma and liver fatty acid profile in mice. The outcome of this study may have implications in biomedical applications for dietary suggestions of lutein with specific lipid for AMD.
Materials and methods
Chemicals and materials
Lutein was extracted and purified (99%) from marigold petals for animal feeding studies. Standard lutein (99%), butylated hydroxyl toluene (BHT), pepsin (porcine gastric mucosa, 88–2500 units/mg protein), bile extract from porcine, pancreatin (porcine pancreas, 8 × U.S.P. specifications) and fatty acid standards were purchased from Sigma–Aldrich (St. Louis, USA). Analytical grade methanol (MeOH), dichloromethane (DCM), acetone, ethyl acetate, diethyl ether, chloroform, silica (60–120 mesh), ammonium acetate, HPLC grade acetonitrile, MeOH, and DCM were purchased from Sisco Research Laboratories (Mumbai, India). Soy lecithin (99%, α-phosphatidylcholine) was purchased from Himedia laboratories (Mumbai, India). Groundnut oil was purchased from local super market (Mysore, India).
Extraction and purification of lutein from marigold flower
Lutein was extracted and purified from marigold petals as per Lakshminarayana et al. 6 with slight modification. In brief, fresh marigold petals (10 g) were ground well along with sodium sulfate (5 g) and 0.1% BHT. Total carotenoids were extracted in ice-cold acetone, saponified with potassium hydroxide (30%) at dark for 3 h and phase separated with hexane. The extract was evaporated using rotary evaporator (Buchi, Switzerland) and the residue was applied on to a open column chromatography (OCC, 20 cm × 1.5 cm) packed with activated silica gel (particle size 60–120) for purification of lutein by using DCM/MeOH (1:1 v/v). The purity of lutein was quantified by HPLC, based on the peak area of standard lutein at 450 nm.
Extraction and purification of lipids
Total lipid (crude lipid) was extracted from the germinated wheat as per Folch et al. 11. Glycolipid was fractionated from the crude lipid as per Sugawara and Miyazawa 12 with slight modification. In brief, the lipid was extracted (three times) with acetone/MeOH (7:3 v/v), concentrated using rota evaporator at 37°C, redissolved in ethyl acetate and phase separated using ethyl acetate/water (1:1 v/v). The ethyl acetate fraction was dried, concentrated, and re-dissolved (10 mL) in hexane/ethyl acetate (9:1 v/v). Further, glycolipid was purified by OCC (20 cm × 3cm) on silica gel (60–120 mesh) eluting with acetone after the removal of neutral lipids with chloroform. Groundnut oil was used to purify neutral lipids using OCC with chloroform. Total lipids extracted from germinated wheat were treated as crude lipid.
Lutein micellarization – in vitro digestion method
To determine the role of glyco-, phospho-, and neutral lipids on lutein micellarization in vitro, purified lutein (600 nmol) was dispersed in respective lipids (50 mg) in a 15 mL screw cap test tube (n = 3). The samples were subjected to in vitro digestion simulating gastric and intestinal phase of digestion as per Garret et al. 7. A separate test tube with no added lipid was run simultaneously (control). In brief, 3 mL of 0.5% pepsin solution (pH 2) in phosphate buffer (3.6 mM CaCl2, 1.4 mM MgCl2 · 6H2O, 49 mM NaCl, 12 mM KCl, 6.4 mM KH2PO4) was added to lutein–lipid mixture (pH, 2.02). The tubes were incubated at 37°C for 1 h in a shaking water bath (Scigenics Orbitek, India) at 120 strokes/min (gastric phase). On cooling, 0.1 M NaHCO3 (6 mL) containing pancreatin (8 g/L) and bile extract (12.6 g/L) were added and the pH of the digesta was adjusted to 7.5 with 1 N NaOH. The test tubes were incubated at 37°C with shaking at 120 strokes/min for 2 h (intestinal phase). After incubation, 1 mL of digesta from each sample was centrifuged (Z 360 K, BHG Hermle, Gosheim) at 12 000 × g at 4°C for 120 min to separate the aqueous fraction that contain lutein micelles. Lutein was extracted and quantified by HPLC to determine the micellarized lutein.
Preparation of mixed micelles and lipid vesicles of lutein
Mixed micelles and lipid vesicles were prepared in phosphate buffered saline 6 containing monooleoyl glycerol (2.5 mM), oleic acid (7.5 mM), sodium taurocholate (12 mM), cholesterol (0.5 mM), and lutein (200 µM) with glyco-, phospho-, neutral, crude lipid extract (3.45 mg), or control micelles (mixed micelles with no phospho-, glyco-, neutral, or crude lipid). Appropriate concentration of chemicals and lipids were dissolved in chloroform and mixed to reach the final concentration. The solvent was evaporated to dryness using nitrogen and the mixture was suspended in phosphate buffered saline (pH 7) with vigorous mixing using a vortex mixer and sonicated (PCI, Mumbai) for 30 min to obtain a clear solution 13 (except the one contained crude lipid).
Analysis of the structure and the approximate size of the mixed micelles and of the lipid vesicles of lutein
The structure and approximate size of lipid vesicles with lutein were characterized with atomic force microscope (AFM, Nanosurf AG, Switzerland). In brief, a thin film of sample (100 µL) was coated on a glass slide and was allowed to dry for 10 min. The slides were scanned with AFM and Nanosurf Easyscan-2 software was used for AFM analysis.
Animal experiment
Animal experiments were performed after due clearance from the institutional animal ethics committee. Male albino mice [OUTB/Swiss Albino/IND/CFT (2c)] weighing 24 ± 2 g, were housed (n = 30) in individual stainless steel cages at RT (28 ± 2°C) with a 12 h light/dark cycle in the institute animal house facility, and received pellet diet (Sai Durga Feeds, Bangalore, India) and had free access to water. After acclimatization (7 days), mice were deprived of food for 12 h before administration of lutein solubilized in mixed micelles or lipid vesicles containing glyco-, phospho- and neutral or crude lipids. Diet, control micelles, and lipid vesicles were processed for lutein analysis to ascertain its level.
Gavage studies
Group of mice (n = 5/group) were intubated a dose of lutein solubilized in glyco-, phospho-, neutral, crude lipid vesicles, or control micelles. A separate group (n = 5) not fed micelles/lipid vesicles was considered as baseline. In order to minimize the usage of animals, blood (100 µL) was drawn using heparinized capillaries (Hirschmann laborgerate GmbH & co., Germany) from the caudal vein 14 into heparinized tubes after 0, 2, and 4 h of gavage of lutein, without sacrificing the animal. At the end of 8 h, animals were sacrificed; blood was drawn directly from heart into heparinized tubes and centrifuged at 1000 × g for 15 min at 4°C to obtain plasma. The liver, intestine, and eyes were excised, washed with ice-cold isotonic saline, and stored at −70°C until analyzed.
Extraction of lutein from digesta, plasma, and tissues
Lutein was extracted from plasma according to Lakshminarayana et al. 6. Briefly, 50 µL of plasma was made up to 100 µL with saline followed by addition of DCM/MeOH (240 µL, 2:1 v/v) containing α-tocopherol (2 mM) and vortexed for 1 min. To the mixture, hexane (120 µL) was added, mixed well, centrifuged at 1000 × g for 5 min, and the upper hexane/DCM phase was collected. The extraction procedure was repeated thrice with DCM (120 µL) and hexane (240 µL). The extracts were pooled, evaporated to dryness using nitrogen, redissolved in 100 µL of acetonitrile/MeOH/DCM (60:20:20 by volume, 0.1% ammonium acetate, mobile phase) for HPLC analysis.
Liver, eyes (pooled samples, n = 5 mice), intestine (8th hour sample), and feed samples were homogenized (Potter-Elvehjem homogenizer, Remi Instruments Ltd. Mumbai, India) separately with nine parts of ice-cold isotonic saline. In case of intestine mucosal layer (duodenum to jejunum) was scrapped using a cover glass, the mucosa was homogenized to form a uniform phase and used for extraction. Lutein was extracted from homogenates (0.8 mL) and digesta (1 mL) as per Lakshminarayana et al. 6. In the case of liver, samples were saponified separately with 2 mL of 10 M potassium hydroxide at 60°C for 45 min and vortexed every 15 min during saponification with an addition of 2 mL of ice-cold deionized water before lutein extraction. Sample handling, homogenization, and extraction were carried out on ice under dim yellow light to minimize light induced isomerization and oxidation of lutein.
HPLC analysis
Lutein extracted from the plasma, tissues, digesta, and feed samples was quantified with an HPLC system (LC-10A; Shimadzu, Kyoto, Japan) equipped with photodiode array detector (SPD-M20A, Shimadzu). Lutein was separated on a Princeton SPHER C-30 (ODS) column (250 mm × 4.6 mm; 5 µm) isocratically eluting with 1 mL/min of mobile phase at 450 nm (Shimadzu Class-VP version 6.14 SP1 software). The peak identity of lutein and zeaxanthin was confirmed by its characteristic spectrum and was quantified by comparing their peak area with authentic standards. Lutein being isomerized to zeaxanthin, lutein was quantified as lutein + zeaxanthin.
Analysis of fatty acids
Total lipid was extracted from plasma and tissues (8th hour sample) as per Folch et al. 11. Fatty acid composition of glyco-, phospho-, neutral, crude lipids, plasma, and liver samples were analyzed as methyl esters prepared using boron trifluoride in methanol 15 and analyzed by GC (Perkin Elmer, fitted with FID) using fused-silica capillary column (25 cm × 0.25 mm) (Parma bond FFAP-DF-0.25: Machery-Nagel Gm BH co. Duren, Germany). The initial column, injector, and detector temperature were set at 160, 250, and 250°C, respectively. Column temperature was programmed to rise at 6°C/min to the final temperature of 260°C. Nitrogen gas was used as the carrier. Comparing with retention times of respective reference fatty acids identified individual fatty acids.
Statistical analysis
To quantify the postprandial plasma lutein level over 8 h, area under the curve (AUC) was calculated by trapezoidal approximation. Data were tested for homogeneity of variances by the Bartlett test. When homogenous variances were confirmed, the data were tested by ANOVA and significant differences between the groups were evaluated by Tukey's test. Differences in means were considered significant at p<0.05.
Results
The purity of lutein and zeaxanthin obtained from the extract of marigold petals by OCC was 97 ± 2% and their λmax was 445 and 452 nm. The total lipid content of germinated wheat was 1.2% (dry wt. basis) in which the levels of glyco-, phospho-, and neutral lipids were 30, 10, and 50%, respectively. The purity of neutral lipid obtained from groundnut oil was 98%. Fatty acid profile of these purified lipids is given in Table 1. The major fatty acids in glyco-, phospho-, and neutral lipids were linoleic acid (52, 45, and 16%) and oleic acid (18, 16, and 51%) whereas, in the crude lipid, linoleic acid (56%), and palmitic acid (19%) are the major fatty acids.
| Fatty acids (%) | Purified lipids | Plasma | Liver | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL | PL | NL | CL | Control | GL | PL | NL | CL | Control | GL | PL | NL | CL | |
| 16:0 | 11 ± 0.1 a | 25 ± 1 a | 17 ± 0.6 a | 19 ± 0.8 a | 36.4 ± 4 a | 40.2 ± 8 b | 46.6 ± 6 b | 33.5 ± 7 c | 38.2 ± 8 b | 30 ± 3.3 a | 30.4 ± 1.6 a | 28.7 ± 4.8 a | 25.92 ± 1.8 a | 29.5 ± 3.7 a |
| 18:0 | 3 ± 0.8 a | ND | 6 ± 0.8 b | 1 ± 0.1 c | 12.5 ± 1.4 a | 19.1 ± 2.2 b | 20.7 ± 3.5 b | 18.4 ± 0.8 b | 17.6 ± 0.7 b | 7 ± 1.5 a | 5.4 ± 0.9 b | 8.4 ± 1.7 b | 7 ± 2.2 a | 6.7 ± 2.1 a |
| 18:1 (n − 9) | 18 ± 1.2 a | 16 ± 0.5 b | 51 ± 0.5 c | 16 ± 1 a | 22 ± 2 a | 24.1 ± 4.1 b | 18.5 ± 2.4 b | 15.9 ± 3.4 c | 11.5 ± 3.1 d | 26.6 ± 0.5 a | 25.7 ± 3.3 a | 25.7 ± 3 b | 30 ± 5.6 a | 27 ± 5.4 a |
| 18:2 (n − 6) | 52 ± 1 a | 45 ± 1 b | 16 ± 0.6 c | 56 ± 0.9 d | 21.5 ± 6 a | 10.2 ± 2.7 b | 10.5 ± 2.3 b | 10.7 ± 3.4 b | 21.5 ± 6.1 a | 24.6 ± 0.7 a | 23.1 ± 1.6 a | 21.1 ± 1.8 b | 23.8 ± 2.6 a | 20.3 ± 1.3 b |
| 20:4 | ND | ND | 1.8 ± 0.2 | ND | ND | 2.2 ± 1.2 a | ND | ND | 7.56 ± 4 a | 2.1 ± 1 a | 2.76 ± 0.4 a | 2.95 ± 0.4 a | 2.3 ± 0.9 a | 5.05 ± 1.8 a |
| SFA:UFA | 0.2 | 0.41 | 0.32 | 0.27 | 1.15 a | 1.72 b | 2.32 c | 1.94 d | 1.69 b,d | 0.67 a | 0.68 a | 0.74 a | 0.57 a | 0.70 a |
| (48.3) | (100) | (67.2) | (45.7) | |||||||||||
| Unidentified FA | 18 ± 3 | 4 ± 0.8 | 10 ± 1.2 | 8 ± 2 | 7 ± 0.5 | 6 ± 1.2 | 4 ± 1.1 | 20 ± 3 | 11 ± 1 | 9 ± 0.2 | 12 ± 2 | 13 ± 2 | 11 ± 1 | 12 ± 1 |
- GL – glycolipid; PL – phospholipid; NL – neutral lipid; CL – crude lipid; SFA:UFA – saturated:unsaturated fatty acids. Values are mean ± SD (n = 5/group. ND – not detected). Values given in the parenthesis are percent difference over control and values in each row not sharing a common letter are significantly different (p<0.05).
Structure and particle size of lipid vesicles
The structure and approximate size of lipid vesicles with glyco-, phospho-, and neutral lipids are shown in Fig. 1. Although the structures of lipid vesicles with those lipids are almost similar, the size range of glyco- (upto 3.43 µm) and phospholipid (upto 5.78 µm) vesicles were significantly smaller than the neutral (upto 66 µm) lipid vesicles.
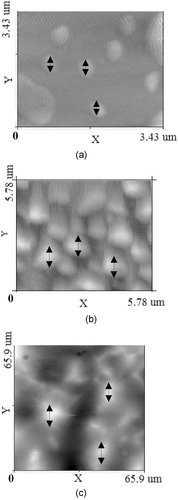
Atomic force micrographs of glyco-(a), phospho- (b), and neutral (c) lipid vesicles with lutein showing their morphology and approximate size (magnification = 100 X × 70X).
Micellarable lutein in vitro
Simulated digestion in vitro was used to find out the influence of lipids on lutein micellarization, which was available for intestinal absorption. The percent micellarized lutein after intestinal phase of digestion was higher in crude (67 ± 10%), glyco- (51 ± 8%), phospho- (45 ± 5%), and neutral (39 ± 2%) lipids than control (Fig. 2). Further, the micellarized lutein in crude and glycolipids were higher by 91.4 and 45.7% than control while no significant difference was observed between phospho- and neutral lipids (28.3, 12%). Results show that the percent micellarization of lutein was in the order of crude lipid>glycolipid>phospholipid>neutral lipid.
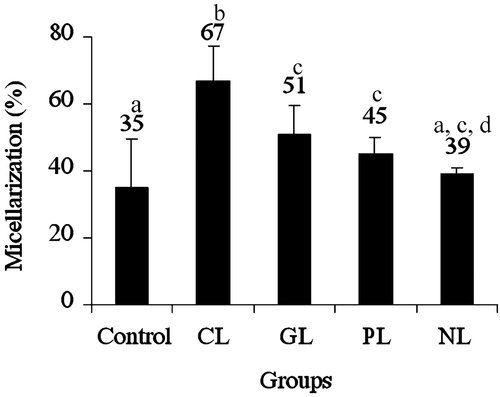
Percent micellarization of lutein solubilized in different lipids under simulated digestion in vitro. Values not sharing a common letter are significantly different (p<0.05) between the groups as determined by one-way ANOVA followed by Tukey's test. CL – crude lipid, GL – glycolipid, PL – phospholipid, NL – neutral lipid.
Lutein bioavailability in vivo
Representative HPLC chromatograms of lutein extracted from plasma (4 h), eyes (8 h) and intestine (8 h), liver (8 h) of glycolipid fed group were shown in Fig. 3. Lutein and zeaxanthin were separated at 7.7 and 9.2 min while they were not detected in the plasma of mice at 0 h. After gavages, maximum lutein levels in plasma (nM) were reached maximum at 4 h in glyco- (6.5 ± 1.8), phospho- (7.3 ± 6), crude (22.3 ± 8.7) lipid, and control (3.8 ± 0.8) groups and at 2 h in neutral lipid (4.7 ± 0.2) group (Table 2). The lutein clearance from maximum postprandial lutein peak was slower (1 h) for glycolipid than phospholipid (2.3 h), neutral lipid (4.7 h), crude lipid (2 h), and control (2.1 h) groups. The mean AUC values of plasma lutein (nmol/mL 8 h−1) in the glyco- (38.7 ± 15.9), phospho- (33.2 ± 25), crude (88.8 ± 55), and neutral lipid (23.4 ± 17) groups were significantly higher than the control (12.4 ± 1.18) group (Table 2 and Fig. 4). However, the mean AUC values for glyco- and crude lipid groups were higher than phospho- (14, 62%) and neutral (39, 73%) lipid groups.
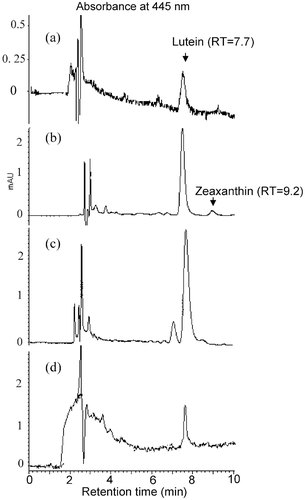
Representative chromatograms of HPLC elution of lutein and zeaxanthin in plasma at 4 h (a), eyes (b) and intestine (c) at 8 h, of mice after gavage of a dose of micellar and lipid vesicles with lutein solubilized in glycolipid. Zeaxanthin is below the detectable limit (1 pmol).
| Groups | Maximum L at ST (nM) | Maximum lutein postprandial peak (h) | L at 8 h (nM) | T1/2 (h) | AUC (nmol/mL 8 h−1) |
|---|---|---|---|---|---|
| Control | 3.8 ± 1 a | 4 | 0.09 | 2.1 | 12.4 ± 1.9 a |
| Phospholipid | 7.3 ± 6 b | 4 | 0.37 | 2.3 | 33.2 ± 25.4 b (167) |
| Glycolipid | 6.5 ± 2 b | 4 | 4.47 | 1 | 38.7 ± 15.9 b (212) |
| Neutral lipid | 4.7 ± 0 a | 2 | 0.85 | 4.7 | 23.4 ± 2 c (88) |
| Crude lipid | 22.3 ± 9 c | 4 | 0.22 | 2 | 88.8 ± 6 d (609) |
- Values are mean ± SD (n = 5 group), value in each column not sharing a common letter are significantly different (p<0.05) between groups determined by ANOVA with Tukey's test. Values given in the parenthesis are percentage change over control. L – lutein, ST – time taken to absorb maximum lutein, T1/2 – time for 50% clearance of absorbed lutein, AUC – area under the curve.
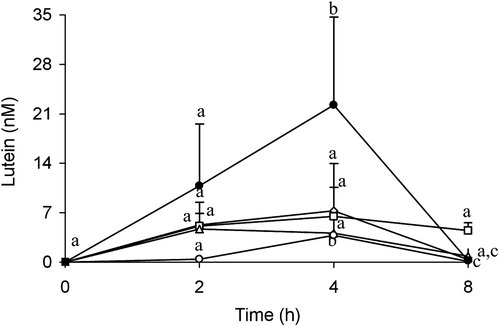
Postprandial plasma response of lutein in mice after a gavage of a dose of micellar and lipid vesicles with lutein solubilized in glycolipid (□), phospholipid (◊), neutral lipid (Δ) crude lipid extract ( ), and control (
), and control ( ). Data represent mean ± SD (n = 5). The lutein values represent as lutein + zeaxanthin. Value at each time point not sharing a common letter are significantly different (p<0.05) between groups determined by ANOVA with Tukey's test after log transformation. Lutein at 0 h was not detected.
). Data represent mean ± SD (n = 5). The lutein values represent as lutein + zeaxanthin. Value at each time point not sharing a common letter are significantly different (p<0.05) between groups determined by ANOVA with Tukey's test after log transformation. Lutein at 0 h was not detected.
Since animals were sacrificed at the end of each experiment (8 h), no time course data are available for liver and eye lutein levels. Hence, data obtained after 8 h gavage of lutein is given in Table 3. Lutein levels (pmol/g) in liver were higher in crude lipid group (7.4 ± 1), followed by phospho- (3.6 ± 0.8), control (2.1 ± 0.2), and glyco- (1.0 ± 0.2) groups. No lutein was detected in the liver of neutral lipid group. Since the lutein level in each eye sample was below the detectable limit (1 pmol), eyes were pooled (10 eyes from 5 mice) and extracted for lutein. The lutein accumulation in eyes (pmol/g) was higher for glyco- (54.0) followed by neutral (21.2), phospho- (19.1), and crude lipid (8.8) groups (Table 3). As in the case of eyes, lutein level in the intestine (pmol/15cm) was higher in glycolipid group (113 ± 10.8) followed by phospholipid (37 ± 3.4), crude lipid (13.1 ± 9.5), and neutral lipid (1.0 ± 1) groups (Table 3). In intestine samples, in addition to lutein, 3′-oxolutein has been identified and that has not been included in the data.
| Groups | Intestinea) (pmol/15 cm) | Liverb) (pmol/g) | Eyes (pmol/g) |
|---|---|---|---|
| Control | ND | 2.1 ± 0.2 a | ND |
| Phospholipid | 37.0 ± 3.4 a | 3.6 ± 0.8 b | 19.1 |
| Glycolipid | 113 ± 10.8 b | 1.0 ± 0.2 c | 54.0 |
| Neutral lipid | 1.0 ± 0.1 c | ND | 21.2 |
| Crude lipid | 13.1 ± 9.5 d | 7.4 ± 1 d | 8.9 |
- a) Comparision is made among lipid group without the control.
- b) Comparision is made among lipid groups except neutral lipid group. Values in each column (except eyes) are mean ± SD (n = 5/group), ND – below the detectable limit (1 pmol), hence considered as zero for calculations. In case of eyes, pooled eyes from 5 mice. Value at each column not sharing a common letter are significantly different (p<0.05) between groups determined by ANOVA with Tukey's test.
On gavage of lutein in different lipid vesicles, the ratio of saturated: unsaturated fatty acids in plasma are higher in phospholipid (2.32) group followed by neutral (1.94), glyco- (1.72), crude (1.69) lipids, and control (1.15) groups while no difference was found between crude lipid and glycolipid groups (Table 1).
Discussion
Purified lutein (97 ± 2%) from marigold petals, crude lipids and glycolipids (30%) from germinated wheat were used in this study. The major fatty acids in the glycolipid were palmitic acid (11%), oleic acid (18%), and linoleic acid (52%) (Table 1). Konopka et al. 16 have reported the glycolipid content differently for spring and winter wheat varieties as 134–215 mg/100 g flour while Sugawara and Miyazawa 12 have reported 226 mg/100 g flour. Slightly higher level of glycolipid (300 mg/100 g) found in the germinated wheat in this study may be due to varietal difference. As found in this study, Prabhasankar and Haridas Rao 17 have also reported that linoleic acid (57%) is the major fatty acid in germinated wheat.
The present study was aimed to understand the effect of glyco-, phospho-, neutral, and crude lipids with varying fatty acids and head groups on lutein micellarization in vitro and in vivo. Results from in vitro study show that micellarized lutein was higher in case of crude lipid, followed by glyco-, phospho-, and neutral lipids than control. Further, the percent micellarable lutein with crude lipid was significantly higher by 91.4% over control while it was moderately higher in case of glyco- over phospho- (13%) and neutral (30%) lipids, respectively. Whereas, Garrett et al. 7 reported that the micellarization of lutein was higher with vegetable oil compared to lipid source from chicken.
The postprandial plasma response of lutein in mice has been used to measure of intestinal lutein absorption (Table 2). As evidenced in vitro, the mean plasma response of lutein was 6-fold higher for crude lipid group followed by glyco- (3-fold), phospho- (2.7-fold), and neutral (2-fold) lipid groups than control (12.4 ± 1.18 nmol/mL 8 h−1) group. Further, the AUC value for glyco-group was higher by 15 and 65% than phospho- and neutral lipid groups, respectively. An interesting observation found in this study was the positive correlation (r = 0.9 (crude), 0.7 (neutral), 0.8 (glyco-), 0.8 (phospho-), and 0.6 (control)) between percentage micellarization of lutein in vitro and plasma AUC of lutein in vivo, which further demonstrate that specific lipids play an important role in the process of intestinal absorption. The higher level of micellarable lutein found in vitro and its response in plasma of crude lipid group may be attributed to the synergistic effect of lipids present in crude lipid. However, bioavailability of lutein was favored by glycolipid although its effect was lower than the crude lipid demonstrating that total lipids are the best for enhancing lipophilic carotenoids. Further, difference in plasma lutein response among lipid groups may also be attributed to the variation in their chemical structure with respect to fatty acids and functional groups 18, 19. The presence of glycoside moiety attached to 1,2-acyl glycerol in glycolipids may help in enhanced bioavailability of lutein due to its hydrophilicity as compared to phospho- and neutral lipid. In contrast, phospholipids with a non-polar fatty acid chain may be less soluble in water compared to glycolipid, which may be the reason for lower plasma lutein level.
Micelles, micro- and nano-emulsions are emerging tools for delivery of bioactive molecules for improved absorption from intestine. The present study hypothesized that lipids with varying functional groups designed for lipid vesicles may influence its architecture and ability to deliver the entrapped materials. Another possible reason for the influential effect of glycolipid on intestinal lutein uptake may be attributed to their smaller micellar size than phospho- and neutral lipids. The size of the lipid vesicles with different lipids is inversely proportional with plasma level of lutein indicating that the size of the lipid vesicles plays a critical role in absorption. In the present study, the size of glyco- (17-fold) and phospholipid (10-fold) vesicles is smaller than the neutral lipid vesicles. Further, Ulrich-Bott and Wiegandt 20 stated that glycolipid micelles are oblate ellipsoid in shape and smaller in size (60 nm). Hollander and Ruble 21 suggested that micelles larger in size decrease the β-carotene absorption in rat. Non-bilayer glycolipids like monogalactosyl diacyl glycerol (MGDG) assemble into the hexagonal phase as they have a conical shape with a small polar head group and bulky acyl moieties. This may be due to the presence of higher levels of unsaturated fatty acids that reported to increase the hexagonal phase of glycolipid, which may favor the carotenoid bioavailability 22. It is hypothesized that the hydrophobic acyl chain of phospholipid expose to the membrane surface by reducing the packaging density in the polar head group. Thus, it is plausible that glycolipid incorporated into the intestinal cells may enhance lutein transfer by inserting a hydrophobic area into the surface of the enterocyte. Further, glycolipid from wheat contains both MGDG and digalactosyl diacyl glycerol (DGDG) 12, which may synergistically help in greater incorporation of lutein lipid vesicles than phospho-, and neutral lipid vesicles in to the intestinal membrane 23. The present results demonstrate that glycolipid not only help in lutein absorption, but also aid in plasma clearance of absorbed lutein faster (T1/2, 1 h) than phospho- (2.3 h), crude (2 h), and neutral (4.7 h) lipids. This could be the reason for higher lutein accumulation in eyes of the glycolipid group than other groups. In the case of plasma, higher saturated: unsaturated fatty acids ratio in case of phospho- and glyco-group may be attributed to higher lutein levels, but it is not in the case of liver. The higher intestinal levels of lutein in glyco-group, even after 8 h intubation, as compared with other groups, suggest that absorption process is continuing which could be the reason for the higher level of maximum postprandial lutein. Anderson et al. 24 opined that digestion of glycolipids is similar to that of phospholipids and they might be more efficiently hydrolyzed by lipase when they are mixed with hydrophobic dietary lipids. The mechanism by which glycolipid aids in lutein bioavailability and the role of lipase needs thorough study.
Conclusions
The influence of lipids on lutein bioavailability in mice was in the order of crude lipid mixture>glyco>phospho>neutral lipid. Lutein bioavailability was higher for glyco- and phospholipids than neutral lipid group, but the effect was lower than crude lipid. This study suggests that ingestion of lutein with glycolipids or phospholipids is best for eyes, which is of high potential interest to prevent or treat AMD.
Acknowledgements
G. Aruna acknowledges the University Grants Commission, New Delhi, India for granting Senior Research Fellowship.
The authors have declared no conflict of interest.




