Cluster of differentiation 147 complex: A putative early predictive biomarker for preeclampsia?
[Correction added on November 4, 2022 after first online publication: the Author Contributions, Acknowledgments, Funding, Conflicts of Interest, Data Availability Statement and Ethical approval has been updated].
Abstract
Preeclampsia (PE) occurs only in humans and some non-human primates, making its pathogenesis difficult to study. The aetiology of PE is mainly caused by a deficiency in trophoblast differentiation and spiral artery remodelling. The angiogenic factor Placental Growth Factor (PlGF) or the ratio of sFlt-1 to PlGF and cell-free RNA have been used as the main biomarkers for predicting PE. Now, Lee and colleagues show that the deficiency of cluster of differentiation 147 (CD147) results in preeclampsia pathophysiology. This contribution makes sheds light on PE development and unveils CD147 as a potential biomarker for clinical trials.
A cluster of differentiation 147 (CD147) also known as Basigin or extracellular matrix metalloproteinase inducer is a member of the immunoglobulin superfamily. CD147 plays an important role in a variety of healthy and diseased cells and tissues. Recently, a new signalling pathway has been revealed explaining the mechanism of CD147 regulation in different cancers. In addition, CD147 was also found to have a negative regulatory role in the T-cell-mediated immune response.1
Extensive literature has exposed CD147 interaction with a variety of proteins regulating metabolism, development, and intercellular recognition. These ligands include Integrin β1, Ubiquitin C, MCT1 and other important proteins in the human body.2, 3 This enables CD147 to directly influence important physiological processes such as calcium signal transduction, angiogenesis, and lactate metabolism.
CD147 is an important molecule in reproductive processes, including fertilization, implantation, angiogenesis, and delivery. Early studies reported that increased expression of CD147 in the endometrial epithelium was observed in the embryonic junction of mice. Despite CD147-deficient embryos developing normally prior to implantation, they could not be successfully implanted, hindering further experimental studies in this animal model. Here, Lee and colleagues knock down CD147 to avoid infertility in mice but still affect embryo development. CD147 knockdown mice showed peripheral somatic voluteal artery remodelling functions, leading to Preeclampsia (PE). The authors also found that the Wnt β-catenin expression pathway was affected by CD147 knockdown. This led to the conclusion that the lack of CD147 may affect embryonic development by inhibiting the expression of the downstream pathway Wnt.
PE is a pregnancy disease, usually caused by placental dysfunction.4, 5 It is the third leading cause of maternal death in the United States. PE impacts an estimated 2%–8% of pregnancies worldwide and leads to 10%–15% of maternal deaths. PE affects both the mother and the fetus.5 In severe cases, the mother may present erythrocyte decomposition, low platelet count, impaired liver function, renal dysfunction, swelling, shortness of breath or visual impairment caused by lung fluid.6
Adequate fetal development requires an appropriate intrauterine environment. PE affects the normal growth and development of the fetus. Serious complications include fetal growth restriction/intrauterine growth restriction, oligohydramnios and increased risk of perinatal death. Risk factors for PE include obesity, past hypertension, old age and diabetes; and if not treated in time, it can lead to epilepsy in the fetus, which is called eclampsia.
Primary pre-production begins after 20 weeks of pregnancy. During implantation of the fertilized egg, trophoblastic cells enter the maternal uterine tissue and remodel the uterine artery to establish an adequate blood supply for the normal development of the fetus and placenta. Low expression of CD147 affects a variety of pathways such as Wnt/β-catenin and matrix metalloproteinase (MMP), which are related to the regenerative function of trophoblastic cells, resulting in abnormal cell differentiation and metabolism (Figure 1).
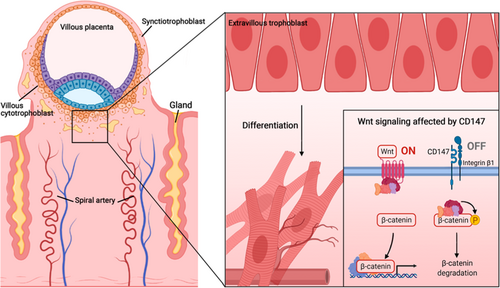
CD147 affects many pathways, among which the Wnt/β-catenin is involved in the differentiation of trophoblast cells. In the absence of CD147, trophoblast cells cannot invade embryos, resulting in the occurrence of preeclampsia. Therefore, low CD147 expression in clinical data may correlate with preeclampsia and be used as a biomarker.
During the implantation of fertilized eggs, trophoblast cells will enter the maternal uterine tissue and reshape the uterine artery to establish an adequate blood supply for normal fetal and placental development. The placenta of peripheral somatic voluteal artery remodelling function of CD147 knockdown mice is impaired, which is supposed to be the main reason for PE.
Recently, Lee and colleagues showed that CD147 was highly expressed on human and mouse placenta; and CD147 down-regulation, with anti-CD147 morpholino antisense oligonucleotides in plCSA-BP-coated nanoparticles, induced preeclampsia pathophysiology in a mice model. Moreover, they found that the numbers of invasive trophoblast giant cells in the CD147 knockdown mice were decreased. Single-cell transcriptomics also showed that CD147 was highly expressed on the cytotrophoblast of human chorionic villus and extravillous trophoblast (EVCT). In addition, blocking CD147 with an antibody suppressed ECVT differentiation from human trophoblast stem cells (TSC) and human trophoblast organoids. This suggested that CD147 regulated the function of trophoblast.
Trophoblast invasion is a highly complex and controlled process, which involves many regulatory mechanisms, such as proteases, adhesion molecules and signalling pathways.7, 8 Lee and colleagues revealed that CD147 promoted EVCT invasiveness by testing the MMP2 expression or activity in the JEG-3 cells. Furthermore, they found that CD147 strengthen the angiogenic ability, showcasing the role of spiral artery remodelling. Importantly, this research group further proved that CD147 binding integrin β1 with Wnt/ β-catenin signalling regulated the EVCT invasion. Finally, the authors performed studies in normotensive and PE women showing that CD147 was decreased in the serum and villi of preeclampsia.
There are many differences in mammalian placental physiology, notably the level of trophoblast invasion into the uterus and the number of cell layers between the maternal and fetal circulation. The mouse and non-human primates may not perfectly reflect the human trophoblast differentiation.9 Lee and colleagues explored the mechanism of CD147 in the placenta by two models: trophoblast organoid and human TSC. The rat placenta possesses similar features to humans, where trophoblast cells deeply invaded to uterine for spiral artery remodelling.10 Therefore, further studies are needed that knockdown CD147 in rats, which should better define the mechanism of CD147 in PE.
In summary, Lee and coworkers elegantly showed that CD147 deficiency in trophoblast is the cause of PE and that this marker is reduced in the serum CD147 in PE patients. In the future, they may wish to screen CD147 to predict PE in numerous asymptomatic women and correlate it to PIGF expression to validate CD147 as a biomarker of PE.
AUTHOR CONTRIBUTIONS
Dr. Wenna Chi and Mr. Yuming Zhang contributed to the preparation and collection of original literatures and figures and the writing and editing of manuscript. Dr. Ligong Chen and Dr. Jinliang Yang were responsible for the structural designs, scientific quality and writing.
ACKNOWLEDGEMENTS
Not applicable.
FUNDING INFORMATION
This work is supported by the Ministry of Science and Technology of China National Key R&D Programs (2022YFA0806503, 2018YFA0506903 to L.C.), National Natural Science Foundation of China (32130048, 92157301, 31971085 and 91857108 to L.C.), Tsinghua University Spring Breeze Fund (2021Z99CFY012 to L.C.), Tsinghua-Foshan Innovation Special Fund (TFISF, 2020THFS0133 to L.C.), Tsinghua Precision medicine foundation (No.2022TS013).
CONFLICT OF INTEREST
The authors declare no conflict of interest. The paper was handled by editors and has undergone a rigorous peer-review process. Dr. Jinliang Yang and Dr. Ligong Chen were not involved in the journal's review of/or decisions related to this manuscript.
ETHICAL APPROVAL
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




