Therapeutic potential of the annexin A family in atherosclerosis
Abstract
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of mortality and morbidity worldwide despite advancements in therapeutic options for the management of atherosclerosis (AS). Treatments that lower low-density lipoprotein (LDL) cholesterol levels, such as statins or proprotein convertase subtilisin/kexin type 9 inhibitors, have effectively reduced ASCVD risk. However, residual CVD risk remains high, highlighting the need for additional effective therapies. Recently, colchicine has been approved for managing AS, introducing new avenues for targeting inflammation, a key process in AS.
Various factors contribute to AS progression, such as endothelial dysfunction, leukocyte transmigration, vascular smooth muscle cell migration and phenotype-switching, increased lipid retention, production of pro-inflammatory cytokines and regulated cell death processes such as apoptosis. The annexin A (AnxA) family of proteins is well-known for their ability to bind Ca2+ and phospholipids, and they play diverse roles in inflammation, cell proliferation, migration, differentiation and signalling. Several AnxA proteins have been implicated in essential processes involved in AS development, including endothelial dysfunction, leukocyte transmigration and apoptosis.
In this mini-review, we highlight the roles of AnxA1, AnxA2, AnxA5, AnxA6, AnxA7 and AnxA8 in AS development and progression and their therapeutic potential in AS management.
1 INTRODUCTION
Atherosclerotic cardiovascular diseases (ASCVD), including ischemic heart disease and stroke, are leading causes of mortality and morbidity worldwide.1 Atherosclerosis (AS) is a complex, chronic inflammatory disease.2, 3 The pathophysiology of AS is complex and involves various cell types and pathways, including endothelial cell (EC) dysfunction, lipid retention and oxidation, vascular smooth muscle cell (VSMC) migration, proliferation, and dedifferentiation, macrophages (Mϕ) infiltration, foam cells formation from Mϕ and VSMCs, inflammatory stimulation, and cell apoptosis and necrosis.2, 3 This complexity presents various cell types and pathways as potential therapeutic targets to slow disease progression.
Standard management of AS includes lipid-lowering agents such as statins, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, ezetimibe and bempedoic acid; antiplatelet drugs like aspirin; beta-blockers (e.g., atenolol, bisoprolol); and renin-angiotensin-aldosterone system inhibitors, including ACE inhibitors.4 Statins and PCSK9 inhibitors work by increasing the levels of low-density lipoprotein receptor (LDLR), thereby reducing LDL cholesterol levels and decreasing the risk of CVD.5, 6 Despite these treatments, significant residual CVD risk remains,7 indicating that targeting multiple mechanisms is necessary. Contributing factors to residual CVD risk include inflammation, pro-thrombotic states and metabolic dysfunction. Clinical trials have targeted inflammation, including interleukin-1β (IL-1β) and broad-spectrum anti-inflammatory medications like colchicine, which the FDA approves to manage CVD in select patients.8 While IL-1β inhibition reduces CVD risk, it is also associated with an increased risk of infection, leading to ongoing debates about its implementation in AS management.9
Annexins are differentially expressed in various cell types and tissues.10 They play an essential role in diverse processes related to AS development, such as inflammation, autophagy and apoptosis, which makes them promising targets for managing AS. In this mini-review, we will discuss the current understanding of annexins, focusing on the role of annexin A (AnxA) in AS and its potential in AS treatment.
2 ANNEXINS: OVERVIEW
Annexins are highly conserved proteins that bind Ca2+ and phospholipids, and are expressed in nearly all eukaryotes and some unicellular eukaryotic organisms. Annexins are classified into five classes (A–E) based on the biological group in which they are expressed. AnxAs are expressed in vertebrates and consist of 12 members: AnxA1, A2, A3, A4, A5, A6, A7, A8, A9, A10, A11 and A13.10, 11 Each AnxA contains a core domain and a diverse N-terminal domain (Figure 1). The core domain consists of four annexin repeats, except for AnxA6, which has eight repeats. Each repeat contains five α-helices connected by short loops.11 Annexins in this family are characterised by their Ca2+-binding capability and a similar core domain and 3D structure.
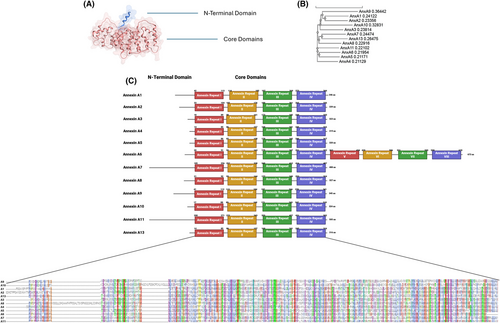
AnxAs play important roles in various physiological functions, such as cell growth and differentiation, membrane trafficking, membrane organisation, exocytosis and endocytosis.12 Although primarily cytosolic, some can be detected on the cell surface or may translocate to the nucleus.13 For example, AnxA1 is transported to the cell surface in response to glucocorticoids, while AnxA2 is constitutively presented on the cell surface of vascular ECs.14, 15 Additionally, AnxA11 and AnxA2 have been found in the nucleus.13 Studies on annexin knockout mice indicate that AnxA1, AnxA2, AnxA5, AnxA6, AnxA7 and AnxA8 play significant roles in the development and progression of AS.
3 ANNEXIN A FAMILY: ROLE IN ATHEROSCLEROSIS
3.1 AnxA1
AnxA1 is a 37 kDa mediator of glucocorticoids that signals through the G protein-coupled receptor lipoxin A4 receptor (ALX) or formyl peptide receptor 2 (FPR2). Known for their anti-inflammatory properties, AnxA1 and its peptides inhibit the transmigration and infiltration of polymorphonuclear leukocytes (PMN), reduce the production of inflammatory mediators, promote apoptosis in neutrophils, and facilitate efferocytosis by Mϕ.16 AnxA1 is secreted upon neutrophil adhesion to the endothelium, promoting neutrophil detachment and inhibiting their vascular transmigration.17 Its inhibitory effect on neutrophil transmigration is mediated through the interaction between FPR and the N-terminal domain of AnxA1 containing amino acids 2–26 (Ac2–26).18 AnxA1 also inhibits changes in adhesion receptors L-selectin and CD11b/CD18 on activated PMN leukocytes,19-21 reduces IL-6 expression, increases IL-10 expression,22, 23 and activates Ca2+ independent phospholipase A2. Effete neutrophils secrete AnxA1, driving chemotaxis of monocytes to the site of inflammation.24 Furthermore, AnxA1 promotes Mϕ polarisation towards the anti-inflammatory M2 phenotype.25 Therefore, AnxA1, as a pro-resolving protein, exerts anti-inflammatory effects via diverse mechanisms, making it a promising target for AS.
In AS, adhesion molecules expressed in activated ECs, such as E-selectin, P-selectin, ICAM-1, and VCAM-1, as well as those in activated leukocytes like L-selectin, increase leukocyte adhesion and subsequent transendothelial migration, thereby promoting inflammation and AS progression.2 Elevated AnxA1 expression in asymptomatic human carotid atherosclerotic plaques is associated with reduced inflammation in VSMCs.26, 27 Similarly, plasma levels of AnxA1 negatively correlate with atherosclerotic lesion size or neointima formation in Apoe−/− mice.28, 29 These findings indicate a protective role of AnxA1 in AS.
In 2015, three different groups investigated the underlying mechanisms of AnxA1's protective effects on AS using various mouse models. All studies validate the beneficial anti-inflammatory effects of AnxA1. Drechsler et al. reported that AnxA1 exerted an atheroprotective effect through the FPR2–AnxA1 axis that specifically inhibited leukocyte infiltration into lesions. Knockout of AnxA1 or Fpr2 in Apoe−/− mice increased atherosclerotic lesions and the content of Mϕ and neutrophils within the lesions. Administration of the annexinA1 mimetic Ac2–26 significantly reduced early AS development and decreased Mϕ counts in Apoe−/− mice through reducing CCL2- and CCL5-mediated β1 and β2 integrin activation in neutrophils and monocytes, along with diminished activation of the GTPase Rap1.29
Consistently, Fredman et al. demonstrated that Ac2–26-containing nanoparticles significantly improved the characteristics of advanced atherosclerotic plaques in Ldlr−/− mice through an FPR2-dependent mechanism, such as reducing plaque sizes and increasing collagen content within lesions.30 On the other hand, Kusters et al. reported that human recombinant AnxA1 had no significant effect on AS progression in Ldlr−/− mice fed a Western Diet (WD) for 6 weeks. However, administering AnxA1 after 6 weeks of WD significantly reduced AS progression.31 This indicates that AnxA1 does not influence plaque initiation but diminishes established plaques. Nevertheless, AnxA1 and its mimetic Ac2–26 play a protective role at different stages of AS development. This is particularly relevant as patients who seek clinical treatment are usually at an advanced stage of AS with established plaques.
3.2 AnxA2
AnxA2 is a 36 kDa protein detected in the cytosol and on the plasma membrane. In the cytosol, AnxA2 exists as a soluble monomer, whereas on the EC plasma membrane, it forms a tetramer consisting of two AnxA2 and two p11 proteins or S100A10.10 The AnxA2-p11 tetramer enhances tissue plasminogen activator (t-PA) activity and promotes fibrinolysis.14 AnxA2 also acts as a linker between actin and the plasma membrane through binding to F and G-actin, essential in maintaining EC adherence junctions with VE-cadherin and facilitating actin-dependent vesicle transport.32, 33 In addition, AnxA2 is involved in inflammatory processes, such as increasing endothelial activation and inflammation in response to disturbed blood flow.34 AnxA2 also mediates chemotaxis of multiple leukocytes, including monocytes, Mϕ and neutrophils, to inflammatory sites.35, 36 These processes are believed to promote inflammation. Conversely, AnxA2 translocates to the damaged membranes of late endosomes or lysosomes to prevent leakage and subsequent activation of the NLRP3 inflammasome pathway,37 mitigating inflammation. Therefore, AnxA2 appears to have a dual role in inflammation.
In addition, AnxA2 is involved in angiogenesis. Vascular endothelial growth factor (VEGF) upregulates AnxA2, inducing angiogenesis.38, 39 Enhanced angiogenesis within plaques increases plaque vulnerability in advanced AS. In conclusion, the complexity of AnxA2's effects may render it pro- and anti-atherosclerotic. It will be interesting to see whether these diverse effects depend on cell types or disease stages, particularly in advanced AS.
AnxA2 has been reported to increase LDLR levels by inhibiting PCSK9-mediated LDLR degradation.40 In Anxa2−/− mice, plasma LDL-C and PCSK9 levels were increased, while LDLR levels were decreased. Consistently, overexpression of AnxA2 increased LDLR levels in wildtype mice but not in Pcsk9−/− mice.41 Given that hepatic LDLR is primarily responsible for the clearance of circulating LDL-C, these findings indicate an anti-atherogenic role for hepatic AnxA2. However, EC AnxA2 has a proatherogenic effect, which involves AnxA2 dephosphorylation by protein tyrosine phosphatase 1B (PTP-1B), a process mediated by disturbed flow activation of the mechanosensitive channel Piezo-1.34 Such modification alters AnxA2 to an open conformation, which allows integrin α5 translocation and activation of several endothelial inflammatory pathways in ECs, thereby exacerbating AS.34, 42 Knockdown of Ptp1b reduced AS progression in Apoe−/− mice but not in Anxa2−/− Apoe−/− mice, indicating the essential role of AnxA2 in this process.34 However, the effects of AnxA2 deficiency on AS development and PCSK9 and LDLR levels in Apoe−/- mice were unclear as they were not investigated in the study.
Recently, Pan et al. showed that knockdown of AnxA2 in Apoe−/− mice reduced AS progression and plasma TG, total cholesterol and LDL-C levels, but increased collagen content, which was due to decreased inflammation.43 Consistently, an in vitro study indicated that AnxA2 promoted inflammation and Mϕ apoptosis.44 However, the reasons behind the discrepancies in lipid profiles observed in the two in vivo studies are unclear. Notably, one study used constitutive Anxa2 global knockout mice, in which Anxa2 was deficient in essentially all tissues from the germline. On the other hand, Pan et al. used Ad-shAnxA2 to silence AnxA2 in adult mice. Adenoviruses preferentially transduce the liver, indicating a possible difference in AnaA2 knockdown across various tissues. Furthermore, shRNA may not reduce AnaA2 expression as efficiently as gene knockout.
AnxA2 also acts as a co-receptor for t-PA and plasminogen in ECs, promoting plasmin production and enhancing fibrinolysis.14 Thrombus formation and platelet activation lead to vessel occlusion, a primary aetiology of stroke, angina pectoris and myocardial infarction.45 AnxA2−/− mice showed increased fibrin deposition in vascular ECs, as well as heightened thrombus formation and occlusion after injury with FeCl3.46 Conversely, administration of recombinant AnxA2 to rats significantly reduced thrombus formation in the same vessel injury model.47 Furthermore, recombinant AnxA2 increased blood flow and reduced infarct size after an autologous clot occlusion in the rat brain.48 Although both studies indicate that AnxA2 may represent an effective thrombolytic therapy for vessel occlusions, its atherogenic effects, particularly from EC AnxA2, could limit its therapeutic potential.
3.3 AnxA5
AnxA5, previously known as annexin V, is a 35 kDa protein that can be found intracellularly and secreted into the extracellular milieu. It has a high affinity for phosphatidylserine (PS), a signal presented by apoptotic cells, which makes AnxA5 valuable for detecting apoptosis.49 PS is essential for the activity of tissue factor and other coagulation factors, including factor Va and VIIa. Additionally, AnxA5 reduces the surface expression of tissue factor, an essential initiator of blood coagulation.50 Thus, AnxA5 was initially recognised as an anticoagulant or antithrombotic protein.51 In addition, AnxA5 exhibits anti-inflammatory effects by interacting with IFN-γ receptor,52 decreasing leukocyte and platelet adhesion to ECs after TNF-α activation,53, 54 reducing TNF-α secretion while promoting IL-10 secretion from Mϕ,54 and enhancing TGF-β and IL-10 production in T cells and subsequently reducing primary T-cell activation.55 It also mitigates inflammation induced by oxidised cardiolipin, a modified phospholipid released from mitochondria during apoptosis and necrosis,56 and reduces ox-LDL uptake in activated Mϕ and their infiltration.57 These effects indicate a beneficial potential of AnxA5 in AS treatment.
Plasma levels of AnxA5 negatively correlate with the severity of coronary AS.58 AnxA5 is also detected in human atherosclerotic plaques, particularly in vulnerable areas.59 Its uptake correlates with AS severity, Mϕ infiltration, and extent of apoptosis in mice, rabbits and humans.49, 60, 61 Conversely, AnxA5 binding to PS in AS is considered a double-edged sword: on the one hand, it can impede the recognition of apoptotic cells, decreasing their effective clearance, but it also promotes apoptotic cell phagocytosis.62 On the other hand, it can suppress thrombus formation. AnxA5 indeed accumulates in areas of active thrombus formation in rats.63 Administration of recombinant AnxA5 increased blood flow and reduced thrombus formation, fibrin deposition and platelet accumulation in rabbits.64 Collectively, these complex functions make AnxA5's role in atherosclerosis elusive but also an interesting target for research.
Ewing et al. evaluated the impacts of AnxA5 on AS in Apoe−/− mice using three different approaches. In a model involving femoral arterial cuff placement, AnxA5 reduced adhesion of leukocytes and Mϕ to ECs, decreased expression of MCP-1 and TNF-α in the artery, and lowered plasma levels of IFN-γ. In WD-fed Apoe−/− mice, AnxA5 improved endothelial function, possibly through NO signalling. Third, AnxA5 reduced vein graft thickening in Apoe−/− mice. However, AnxA5 did not alter Mϕ content, VSMC content or apoptotic cells within the lesion,53 but displayed an anti-inflammatory effect in mice with a vein graft procedure. Furthermore, AnxA5 administration for 3 days following femoral cuff placement in WD-fed Apoe−/− mice reduced leukocyte recruitment in the lesion and decreased neointima formation in a dose-dependent manner.65 Additionally, AnxA5 administration for 4 weeks reduced Mϕ content without affecting lesion size in WD-fed Apoe−/− mice placed with a carotid collar for 5 weeks.54 Notably, Apoe−/− mice that received AnxA5 after 1 week of WD feeding displayed reduced atherosclerotic lesion size and Mϕ content.66 These findings indicate the potential therapeutic role of AnxA5 following percutaneous coronary intervention and highlight its beneficial anti-inflammatory effect in AS, regardless of when the treatment is initiated.
3.4 AnxA6
AnxA6 is the largest member of the annexin family, with a molecular weight of 68 kDa. It is a cytosolic protein that binds to negatively charged phospholipids at the plasma membrane when intracellular Ca+2 levels are elevated.67 The functions of AnxA6 include linking membrane microdomains with cytoskeleton organisation, facilitating membrane repair, promoting exosome secretion, aiding receptor signalling, and transporting cholesterol from late endosomes to lysosomes.67, 68 AnxA6 also facilitates chondrocyte differentiation and mineralisation.69 However, mice lacking Anxa6−/− showed no notable phenotype.70, 71
AnxA6 levels are increased in areas of calcification within atherosclerotic lesions. It enhances mineralisation of VSMC matrix vesicles, increasing calcification, a feature observed in advanced AS.72 Calcification increases the vulnerability and stiffness of lesions, raising the risk of complications.73 VSMCs constitute more than 50% of foam cells in atherosclerotic plaques.74 While contractile VSMCs are beneficial as they increase lesion stability, synthetic VSMCs are detrimental.75 Synthetic VSMCs respond to the inflammatory environment of AS by increasing exosome secretion. These exosomes are enriched with PS, AnxA6 and matrix metalloproteinase-2 (MMP-2), acting as a nidus for calcification. The exposure of PS by these exosomes also increases the risk of coagulation, thereby exacerbating AS.76, 77 Collectively, these data indicate that AnxA6 may promote VSMC calcification within the lesions, potentially exerting an adverse effect.
3.5 AnxA7
AnxA7/synexin is the first annexin discovered and exists in two isoforms weighing 47 and 51 kDa, respectively. It is a membrane-associated protein that binds phospholipids in a Ca+2-dependent manner. AnxA7 possesses intrinsic GTPase activity and is a substrate of protein kinase C.78 It can act as an inducer of autophagy, function as a tumour suppressor or enhancer, and contribute to exocytotic secretion.78, 79 Anxa7−/− mice display altered cardiomyocyte function.80 Notably, AnxA7 increases the levels of LC3-II and induces autophagy in vascular ECs.79, 81 Inhibition of AnxA7 GTPase activity increases the expression of homeobox containing 1 (HMBOX1) transcription factor, further promoting autophagy and inhibiting apoptosis in ECs.82
Apoptosis in ECs increases plaque instability, raising the risk of complications,83 whereas autophagy in ECs is considered atheroprotective.84 Several factors induce apoptosis in AS, including ox-LDL, oxidative damage, hyperglycaemia and hypercholesterolaemia.85 AnxA7 reduces apoptosis and enhances autophagy in ECs by inhibiting the activity of phosphatidylcholine-specific phospholipase C (PC-PLC).79, 86 A small molecule, ABO (6-amino-2,3-dihydro-3-hydroxymethyl-1,4-benzoxazine), can increase AnxA7 levels while inhibiting its phosphorylation and GTPase activity.87 It mitigated atherosclerosis in WD-fed Apoe−/− mice in an AnxA7-dependent manner.86 These findings indicate the potential of targeting AnxA7 in the management of AS, particularly in advanced stages where apoptosis is substantial.
3.6 AnxA8
AnxA8, a 36 kDa protein, was initially identified as an anticoagulant and an inhibitor of phospholipase A2.88 It is involved in cell differentiation and proliferation,89, 90 and VEGF signalling.91 Its deficiency disrupts the association of late endosomes with F-actin, affecting their motility.92 Additionally, AnxA8 is expressed in various cancers and is considered a potential prognostic factor for disease severity or metastasis.93, 94
Unlike other annexins, AnxA8 deficiency exhibits anti-inflammatory activity. For example, AnxA8 knockdown in human umbilical vein endothelial cells (HUVECs) reduced leukocyte adhesion and rolling during inflammation; a similar phenotype was observed in Anxa8−/− mice.95 This effect is attributed to decreased CD63 levels, which leads to lower expression of P-selectin on the EC surface.95 CD63 is a tetraspanin essential for the presentation of P-selectin on ECs.96 AnxA8 deficiency impairs the intracellular trafficking of CD63 from late endosomes, therefore affecting leukocyte adhesion and rolling.92, 95 Notably, decreased expression of P-selectin, E-selectin, and platelet and endothelial cell adhesion molecule-1 (PECAM1) has been noted in ox-LDL-activated Anxa8 knockout primary murine aortic ECs.97 Furthermore, AnxA8 expression is increased in the aortas of Apoe−/− mice compared to WT mice, as well as in human carotid atherosclerotic plaques compared to healthy arteries.97
Given these observed effects of AnxA8 on ECs, Gutiérrez-Muñoz et al. conducted a comprehensive investigation into the effects of AnxA8 deficiency on AS in Apoe−/− mice at different disease stages.97 The lack of Anxa8 in Apoe−/− mice significantly reduced atherosclerotic lesions at both early and advanced stages of AS. These mice also exhibited lower lipid levels and Mϕ content, increased fibrous cap thickness, and reduced necrotic core, indicating more stable atherosclerotic plaques. Additionally, platelet adhesion was decreased due to lower PECAM1 levels.97 Interestingly, apoptosis within the lesions of Anxa8−/− Apoe−/− mice was reduced. Further research is needed to explore whether AnxA8, like AnxA7, regulates apoptosis. Nevertheless, these findings support the role of AnxA8 in mediating the expression of adhesion molecules on the surface of ECs, thereby playing a pro-atherogenic role.
4 SUMMARY AND PERSPECTIVES
Inflammation promotes AS progression, enhancing atherosclerotic plaque vulnerability and complications. Apoptosis also exacerbates plaque instability. Therefore, targeting these processes with AnxA family proteins presents a promising therapeutic approach (Figure 2).
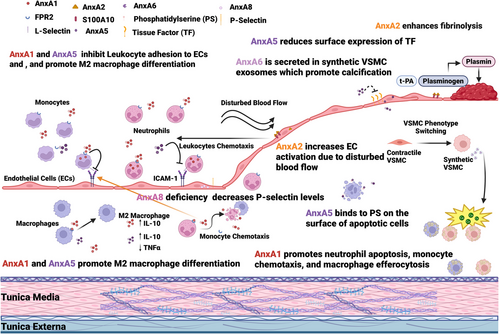
Leukocyte recruitment and transmigration are key drivers of AS progression. AnxA1 and its mimetic Ac2–26 alleviate AS by lowering Mϕ content in lesions, increasing collagen content and fibrous cap thickness, reducing necrotic core and oxidative stress, and decreasing the levels of CCL2 and CCL5.29-31 AnxA5 reduces leukocyte transmigration and inflammation and decreases Mϕ content within lesions.54, 65, 66 AnxA8 inhibition also decreases leukocyte transmigration. However, these effects must be carefully considered in the context of acute infections, where leukocyte recruitment is vital. For example, IL-1β inhibition, despite its benefits in AS treatment, has been associated with an increased risk of sepsis and infections, limiting its use as a treatment option for AS.98 Additionally, patient-specific factors should be taken into account when considering anti-inflammatory therapy, as observed with methotrexate therapy, which fails to manage acute CVD events, probably due to lower baseline c-reactive protein levels in patients.99
Apoptosis contributes to the formation of vulnerable atherosclerotic plaques. The extent of apoptosis in atherosclerotic lesions can be assessed using radiolabelled AnxA5, offering a valuable tool for evaluating disease severity and the risk of complications.61 However, whether this binding can be used to promote effective efferocytosis remains to be investigated. Inhibition of AnxA7 GTPase activity has shown promise in promoting autophagy and preventing apoptosis,86 but further in vivo studies are needed to clarify the underlying mechanisms and therapeutic potential. Additionally, decreased apoptosis within lesions in AnxA8−/− mice warrants further investigation to better understand its molecular mechanism and clinical relevance.97
Thrombosis is a common complication of acute CVD events, occurring when the contents of atherosclerotic plaques are exposed and activate the coagulation cascade. AnxA5, which reduces tissue factor expression, and AnxA2, which enhances fibrinolysis, may serve as acute therapeutic options.14, 50 Still, the effects of AnxA2 on EC activation must be evaluated. Although preclinical studies suggest the potential of annexin-based therapies for AS, no approved or advanced clinical trials exist yet. The modulation of AnxA proteins could lead to innovative strategies for reducing AS complications.
AUTHOR CONTRIBUTIONS
Suha Jarad wrote the first draft of the manuscript. Da-Wei Zhang supervised and directed this project. Suha Jarad and Da-wei Zhang reviewed and wrote the final version of the manuscript.
ACKNOWLEDGEMENTS
This work is supported by a Grant-in-Aid from the Heart and Stroke Foundation of Canada (G-24-0036367). Suha Jarad is partially funded by the Motyl Graduate Studentship in Cardiac Sciences, Faculty of Medicine & Dentistry, University of Alberta.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.




