A snapshot of the role of estrogen-regulated divergent non-coding transcripts
Barbara Yang and Melina J. Sedano contributed equally to this work.
Abstract
Recent high-throughput sequencing technologies have discovered various polymerase II transcribed transcripts. The majority of them are non-protein-coding, understudied and poorly conserved. Non-coding transcripts are categorised based on their location in the genome and the direction in which they are transcribed; these categories classify a non-coding transcript as either antisense, intergenic or divergent. The RNAs belonging to divergent classes consist of two transcripts, transcribed in sense and antisense direction, generated from the same promoter or locus. Multiple environmental and genetic cues can determine the regulation of these transcripts. One of the well-known signalling molecules, estrogen, has been shown to play a vital role in the activation and regulation of divergent transcripts by mediating effects through the estrogen receptors. Emerging studies have shown a strong causative effect between estrogen-regulated divergent transcripts and diseases such as cancer. However, few, viz., lncRNA67, CUPID1 and CUPID2, show a causal relationship with estrogen-dependent biology. This mini-review summarises their role in estrogen-dependent processes that may drive the research to identify novel estrogen-signalling regulators.
1 INTRODUCTION
During transcription in mammals, chromosomal DNA serves as a template for synthesizing complementary RNA transcripts. Initially, the majority of RNA transcripts were thought to function as templates directing protein synthesis. However, later genome-wide studies showed that most transcribed genomes consist of non-protein-coding transcripts, which have high heterogeneity, low expression levels, and primarily unknown functions.1 Non-protein-coding transcripts transcribed by polymerase II (Pol II) are classified based on the genomic region from which they are synthesised in relation to the nearest annotated gene. They can be categorised as enhancer, antisense, divergent, intergenic long non-coding RNAs (lncRNAs), etc.2, 3 (Figure 1). LncRNAs are thought to be mainly expressed at low levels; they are short and unstable, exhibiting diverse conservation patterns across species. Antisense lncRNAs, transcribed from the same locus but in the opposite direction of the annotated RNA, have lesser-known biological roles and are linked to the regulation of gene expression1 (Figure 1). Intergenic lncRNA genes are located between annotated genes.4 Divergent lncRNAs, on the other hand, are typically associated with a more stable RNA transcript in the sense direction and an unstable transcript in the antisense direction of the gene of interest5 (Figure 1). Although the functionality of lncRNAs is still being investigated, they may play a role in enhancer-like activity.6, 7
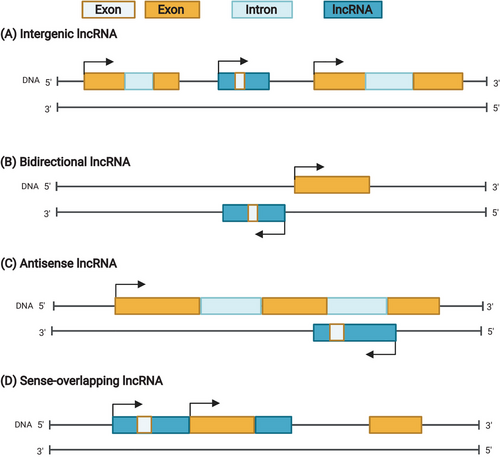
Active transcriptional regulatory elements determine the direction of transcription. They consist of upstream cis-regulatory elements that work in concert with Pol II and other factors, including CpG islands, nucleosome-free regions, histone tail post-translational modifications and chromatin states.5 These elements are crucial for regulating the level and pattern of divergent transcription from bidirectional promoters by modulating transcriptional activity.8 Although genome-wide studies have provided a wealth of information on divergent transcription, little is known about the roles and mechanisms of action of divergent lncRNAs. However, one area for further investigation is the role of hormones such as estrogen, which have been shown to control and modulate the expression of some divergent lncRNAs. Notably, the estrogen receptor (ER) has been implicated in regulating divergent transcripts, underscoring its importance in this process.9 The studies focused on the transcription of divergent transcripts indicate a strong correlation of their expression with the primary transcript in an estrogen-dependent manner.2 As such, divergent transcription and the RNA transcripts arising from bidirectional promoters are crucial for understanding complex biological processes and could yield new diagnostic and therapeutic targets in the ever-evolving landscape of diseases. Investigating the biology of rapidly synthesised divergent RNAs/transcripts in response to signals such as estrogen may uncover intricate details of cellular responses necessary to adapt quickly and robustly to dynamic cellular or tissue environments. Furthermore, examining signal-regulated (specifically estrogen-regulated) non-coding divergent RNAs could elucidate their essential roles within the context of transcription. This review emphasises the significance of three distinct lncRNAs that have been studied to understand their cellular functions in estrogen signalling. We also provided a brief overview of estrogen signalling before delving into specific estrogen-regulated divergent lncRNAs.
1.1 Estrogen signalling
The expression level of target genes can be modified through estrogen signalling, which occurs via the activation of ERs. ERs are found in the cytoplasm and mitochondria but are most abundant in the nucleus. 10-12 These receptors can be activated through various mechanisms but are primarily triggered by the binding of a ligand known as estrogen, which belongs to a class of steroid hormones.2 Upon activation, ERs function as transcription factors to regulate the expression of target genes13-15 (Figure 2). Estrogen signalling, integral to human ‘sexual development, reproduction, cardiovascular and neuronal activity, as well as liver, fat and bone metabolism’, has been identified as a critical player in the dysregulation of endocrine disorders, cancer, cardiovascular disease and osteoporosis.14, 16-18 The estrogen ligand exists in several forms but is most commonly found as the circulating form of estrogen called 17β-estradiol (E2).15 The actions of E2 can be mediated through its association with two possible versions of nuclear ERs, known as ERα and ERβ, or through its binding to a cell membrane ER, designated as the G protein-coupled estrogen receptor (GPER1).19 Several mechanisms of ER signalling exist that facilitate the modulation of estrogen-regulated target gene expression. These mechanisms are categorised based on their effects on expression. Some exhibit genomic effects, where E2‒ER binding directly or indirectly associates with DNA, while others have non-genomic effects, where the initiation of intracellular signalling events ultimately regulates target gene expression.20 Estrogen signalling typically begins with the binding of E2 to a nuclear ER, which activates the receptor, prompting necessary conformational changes, including dimerisation.15, 21 Upon dimerisation, the E2-bound receptor directly binds to specific sequences in the promoters of target genes, referred to as estrogen response elements, and alters local gene transcription levels.15, 21 ER activation and the subsequent change in target gene expression can occur for both protein-coding and non-coding genes.22, 23 Estrogen affects gene expression within minutes, resulting in a steady upregulation of transcripts associated with translation, ribosome biogenesis and protein synthesis—in other words, triggering ‘a mitogenic transcriptional response’.16 Estrogen signalling also plays a role in swiftly, robustly and temporarily regulating genes specific to breast cancer.2, 8, 16
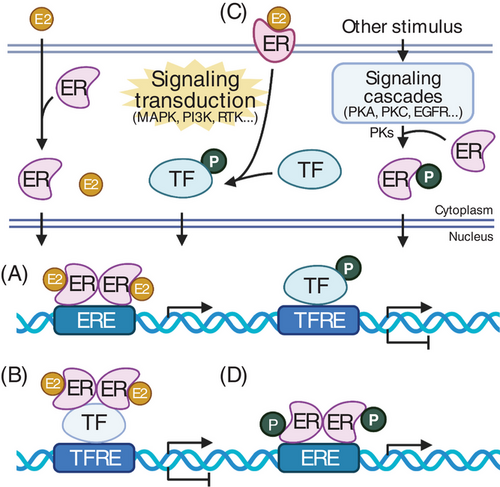
Studies have shown that estrogen, or E2, regulates nearly one-third of all genes, encompassing both annotated and unannotated non-coding transcripts as well as divergent transcripts. E2 treatment of estrogen receptor-positive (ER+) breast cancer cells leads to alterations in the expression levels of lncRNAs.24 This process can contribute to cancer progression when lncRNAs target coding or non-coding genes that regulate critical cellular processes, such as the cell cycle, apoptosis and proliferation.22
Numerous factors and lncRNAs have already been identified, serving as diagnostic markers or therapeutic targets due to their specific expression in certain cell and/or tissue/tumour types. Studies from the past few years show that lncRNAs can function directly as oncogenes or tumour suppressors; additionally, they may interact indirectly with oncogenes or tumour suppressors at both the transcriptional and post-transcriptional levels.25 Over the last decades, several lncRNAs have been identified to be regulated by estrogen. Among those identified, several lncRNAs were found to be responsible for cell cycle progression and proliferation, and aberrant expression of these lncRNAs was linked to the progression of various cancers.26 H19 lncRNA is one of the first identified lncRNAs and is recognised as an oncofetal gene because of its expression during embryogenesis. However, its expression stops after birth; it is also an imprinted gene expressed from the maternal allele.27 Interestingly, H19 has been identified in solid tumours across various cancer types, including breast cancer. Additionally, it is thought to act as an oncogene and is involved in tumour progression.28 Moreover, H19 is regulated by estrogen and affects differentiation and cell proliferation, suggesting that it may serve as a potential biomarker for monitoring breast cancer progression.29 Conversely, some lncRNAs are inhibited by estrogen and play a role in processes such as apoptosis and the silencing of ER signalling.23, 24 One example is enhancer RNAs (eRNAs). Yang et al. identified a group of eRNAs that block estrogen-induced transcription by binding to the DNA-binding domains of ERα. By binding to these sites, cofactors can more easily bind to ERα, which results in the dismissal of RNA Pol II and causes gene silencing.30
Increasing evidence shows that lncRNAs are essential in cancer development, with their expression levels varying significantly between normal and cancer cells.26, 31-35 Endogenous hormones induce developmental and physiological changes in mammals in response to internal and external environmental changes and have been shown to regulate/modify some of these transcripts.17 As mentioned earlier, many are regulated by hormones such as estrogen, which may play crucial roles in signal-dependent cellular processes (Table 1).
| Estrogen-regulated lncRNAs | Response to estrogen | Function | Source |
|---|---|---|---|
| CUPID1 | Upregulated | Controls cell cycle processes, DNA replication, molecular transport, DNA repair | 36 |
| CUPID2 | Upregulated | ||
| MALAT1 | Repressed/downregulated at high E2 concentrations | Nuclear organisation, alternative splicing and epigenetic gene expression; transcriptional regulation | 23, 37, 38 |
| HOTAIR | Upregulated | Involved in metastasis, invasion and progression of some cancers | 38 |
| ANRIL | Upregulated | Regulates estrogen receptor signalling, cellular processes | 39, 40 |
| MIAT | Upregulated | Associated with myocardial infarction susceptibility. Role in E2-induced proliferation and cell cycle progression in ER+ BC cells | 41 |
| BNAT1 | Upregulated | Role in modulating ERα-dependent transcription regulation, proliferation and tumour progression | 42 |
| H19, antisense H19 | Upregulated | Mediates cell proliferation, growth, migration and invasion | 43 |
| LncRNA67 | Upregulated | Divergent transcript; regulates cell proliferation | 27 |
| Lnc01016 | Upregulated | Promotes cell proliferation, migration, decreased apoptosis rate | 44 |
| LncRNA152 | Downregulated | Regulates proliferation and tumour growth | 27, 45 |
- Abbreviations: ER+, estrogen receptor-positive; E2, 17β-estradiol; BC, breast cancer.
Among all the classes of lncRNAs, especially as the association between divergent lncRNAs and estrogen-regulated cancer becomes clearer, further research is needed to understand this category of transcripts and their signalling pathways.
This review focuses on the estrogen-regulated divergent transcripts lncRNA67, CUPID1 and CUPID2.
2 ESTROGEN-REGULATED DIVERGENT LONG NON-CODING RNAS
2.1 LncRNA67
Long non-coding RNA 67 (lncRNA67) is an estrogen-regulated lncRNA that is divergently transcribed from the protein-coding gene programmed cell death 6 interacting protein (PDCD6IP) gene. It was identified using global run-on sequencing (GRO-seq) and RNA sequencing (RNA-seq) in MCF-7 cells.24 LncRNA67 is expressed at significantly higher levels in breast cancer tissue and luminal breast cancer cell lines than in benign breast tissue and normal breast cell lines.24 The GRO- and RNA-seq analyses demonstrated E2-dependent upregulation of lncRNA67 both transcriptionally and at steady state. The small interfering RNA (siRNA)-mediated knockdown of lncRNA67 showed its impact on cell proliferation, which revealed a significant decrease in the growth of ER+ breast cancer cells.24 These findings indicate that lncRNA67 is necessary for breast cancer cell growth and vital for other cell cycle-related processes. It was demonstrated that the knockdown of lncRNA67 affected numerous genes associated with the cell cycle and estrogen signalling. A Genomic Regions Enrichment of Annotation Tools (GREAT) analysis of the downregulated genes revealed enrichment for genes expressed at specific cell cycle stages, potentially targeting the E2F4 transcription factor, which is involved in progressing cells from the G1 to S phase of the cell cycle. Cell cycle analysis was performed to determine at which stage of the cycle lncRNA67 is expressed, and elevated levels were noted during the G1/S phase. When lncRNA67 expression was reduced, cells accumulated in the G1 phase, resulting in fewer cells in the S phase.24 These studies suggest a robust role for lncRNA67 in estrogen signalling, and its noticeable phenotypic effects indicate it could be well used as a diagnostic/prognostic indicator in a clinical setting.
2.2 CUPID1 and CUPID2
CUPID1 (LINC01488) and CUPID2 (LINC02747)(CCND1-upstream intergenic DNA repair 1 and 2) are estrogen-regulated lncRNAs transcribed from a shared bidirectional promoter.36 They were identified by RNA CaptureSeq on breast cancer cell lines when the authors were exploring any lncRNAs transcribed from the 11q13 breast cancer risk locus.36 Locus 11q13 is associated with an increased risk for breast cancer as a single-nucleotide polymorphism rs614367 that lies within the locus, and it is strongly associated with ER+ breast cancer.46, 47 A study conducted by French et al.48 identified a regulatory element located in the 11q13 locus, named putative regulatory element 1 (PRE1), that acts as a distal transcriptional enhancer and regulates the expression of CCND1, a gene encoding cyclin D1. The shared bidirectional promoter of CUPID1 and CUPID 2 maps around 20 kb away from PRE1. It interacts with PRE1, as confirmed through chromatin conformation capture assays in normal breast and breast cancer cell lines, suggesting chromatin looping.36 Luciferase reporter assays using constructs containing only the CUPID1/CUPID2 promoter region, along with constructs that include PRE1, indicate that PRE1 serves as a potent enhancer for the CUPID1/CUPID2 promoters.36
The link between CUPID1/CUPID2 and PRE1 has been further strengthened by experiments employing the dCas9-KRAB method to silence the PRE1 enhancer. The results indicate that expression levels of CUPID1 and CUPID2 are reduced when PRE1 is silenced.36 CUPID1 and CUPID2 are expressed at much higher levels in ER+ breast cancer cell lines than other subtypes of breast cancer or normal breast cell lines.36 When investigating the cellular location of these two lncRNAs, CUPID1 appears to be enriched in the nuclear fraction, while CUPID2 is found in both the nucleus and cytoplasm compartments.36 CUPID1/CUPID2 expression is induced upon estrogen treatment in MCF-7 cells, and PRE1 enhances the activation of the CUPID1/CUPID2 promoter in response to estrogen stimulus. Data from TCGA show that CUPID1 and CUPID2 are frequently expressed in breast tumours, with a quantitative representation of 60.1% and 78.7% of all breast tumours, respectively, and are highly expressed in hormone receptor-positive tumours. TCGA data also suggests a high correlation between the expression of CUPID1 and CUPID2, and this relationship is observed in siRNA-mediated knockdown experiments in ER+ breast cancer cell cells.36
The potential functions of CUPID1/CUPID2 were preliminarily inferred from the same siRNA silencing experiments. Using RNA-seq and ingenuity pathway analysis, it was shown that the suppression of CUPID1/CUPID2 leads to the differential expression of approximately 1800 genes, 362 of which are co-regulated by both lncRNAs. These 362 genes are involved in molecular transport networks, cellular assembly and organisation, DNA replication, recombination and repair. Their role in homologous recombination (HR) DNA repair was experimentally validated. HR repair is inhibited when siRNAs hinder the expression of CUPID1/CUPID2 or when dCAS9-KRAB silences their bidirectional promoter in MCF-7 cells; however, HR DNA repair is restored when CUPID1/CUPID2 are re-expressed in those cells. In parallel, the recruitment of RAD51 to the repair site—a critical step in HR—is affected when CUPID1/2 is knocked down and restored after adding CUPID1/CUPID2 back.36
Interestingly, non-homologous end joining (NHEJ) DNA repair was promoted upon CUPID1/CUPID2 knockdown, likely as a compensating pathway for DNA repair. NHEJ repair, instead of HR repair, can add to the risk of mutations that may eventually lead to cancer development.36 This study highlights the importance of these two novel divergent lncRNAs in breast cancer, particularly in DNA repair mechanisms. Further research involving CUPID1- and CUPID2-dependent effects on DNA repair might present them as potential targets for treating various types of cancer.36
3 CONCLUSION
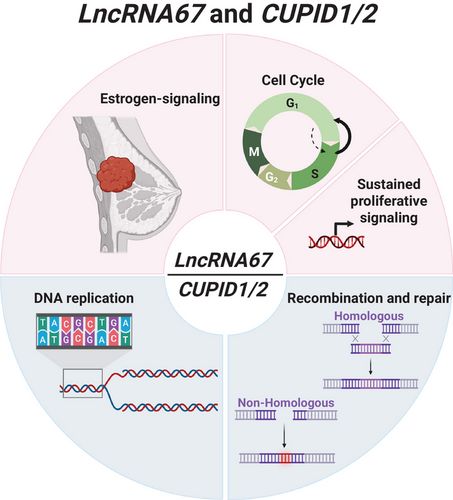
LncRNA67, CUPID1 and CUPID2 are three recently annotated divergent transcripts regulated by estrogen (Figure 3). All play an essential role in cancer development by controlling cell cycle processes, DNA replication and molecular transport. While we have summarised processes and cofactors involved, it is unlikely that these are the only pathways affected by the dysregulation of the expression of these lncRNAs. Identifying divergent transcripts and thoroughly analysing their mechanisms of action, epigenetic effects, and pathways are only the beginning. Functional studies and mouse models are necessary to confirm function, and techniques to identify lncRNA secondary structure could help further assess how mutations could hinder or fortify their overall roles in cellular processes.49 The altered regulation of lncRNA expression in cancer cells is a variance that could serve as a therapeutic approach or a cancer biomarker; thus, taking a closer look at estrogen regulation of different types of transcripts in the non-coding genome can help further unravel cancer biology mechanisms. Moreover, there is a necessity for concerted efforts to identify cellular functions and elucidate the molecular mechanisms through which divergent transcripts control key biological processes. However, studying them is challenging given the way they are synthesised; genetically targeting or manipulating their sequences could affect the primary transcript, potentially obscuring the actual role of the target divergent RNA. Hence, developing new tools or approaches that are aimed at targeting divergent RNA will help distinguish their role from the primary transcript.
AUTHOR CONTRIBUTIONS
Conceptualisation: Shrikanth S. Gadad. Writing—original draft preparation: Barbara Yang, Melina J. Sedano and Alana L. Harrison. Writing—review and editing: Barbara Yang, Melina J. Sedano, Mahalakshmi Vijayaraghavan, Kimberly Diwa, Gabriela Boisselier, Alana L. Harrison, Victoria A. Reid, Enrique I. Ramos, Johnathan Dominguez, Maria V. Jimenez, Laura A. Sanchez-Michael, Shreya Kolli, Jai Patel, Debra Lee, Jessica Chacon, Subramanian Dhandayuthapani and Shrikanth S. Gadad. Supervision: Shrikanth S. Gadad. Project administration: Shrikanth S. Gadad. Funding acquisition: Shrikanth S. Gadad. All the authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
We thank members of the Gadad Lab for their helpful comments. S.S.G. is a CPRIT Scholar in Cancer Research. S.D. is supported by 1RO1AI175837-01. S.S.G. is partly supported by 1RO1AI175837-01, the NIH Lizanell and Colbert Coldwell Foundation, the Edward N. and Margaret G. Marsh Foundation, the American Cancer Society (RSG-22-170-01-RMC) grants and CPRIT-TREC (RP230420). The authors thank Pani and Gadad lab members for their careful review and helpful suggestions on this work. Figures were created with BioRender.com. This work was supported by NIH 1R16GM149497 and a first-time faculty recruitment award from the Cancer Prevention and Research Institute of Texas (CPRIT; RR170020).
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted without any commercial or financial relationships that could potentially create a conflict of interest.
ETHICS STATEMENT
Not applicable.




