Involvement of S100A8 and S100A9 in nonischaemic cardiomyopathy
ABSTRACT
The heterodimeric complex of S100 calcium binding proteins A8 and A9 (S100A8/A9, also known as Calprotectin) is constitutively expressed in myeloid neutrophils and monocytes and plays a role in the modulation of the inflammatory response and cytoskeleton rearrangement. Recently, S100A8/A9 complex has garnered significant attention as a critical alarmin involved in regulating the pathogenesis of various inflammatory cardiovascular diseases, particularly nonischaemic cardiomyopathy (NICM). Furthermore, S100A8/A9 is reportedly associated with the pathophysiological processes of myocardial ischaemia‒reperfusion injury and has also been recognised as a predictor and a potential mediator of heart failure caused by acute myocardial infarction. Recent studies have attempted to provide a comprehensive and detailed overview of the involvement of the S100A8/A9 protein in NICM, covering topics such as hypertrophic myocardial remodelling, septic and dilated cardiomyopathy, myocarditis, chemotherapeutic cardiotoxicity, senescent cardiac dysfunction and cardiac allograft rejection. Ultimately, we aimed to evaluate the application of S100A8/A9 as promising biomarkers and therapeutic strategies for the prediction, prevention and treatment of NICM.
1 INTRODUCTION
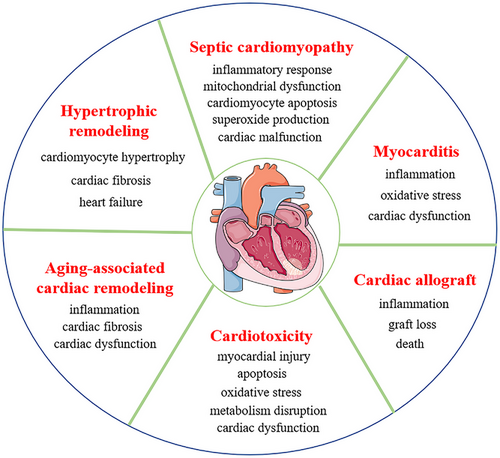
Cardiovascular diseases (CVDs) have been identified as a primary threat to human health and survival because of their rapidly increasing incidence and mortality rates.1, 2 Statistical data demonstrate that the number of CVD patients increased to 523 million in 2019, with 18.6 million deaths annually, which makes CVD the leading cause of death in humans.2, 3 CVDs are a group of ischaemic heart disease (IHD) and nonischaemic cardiomyopathies (NICMs). IHD is caused by an insufficient supply of blood and oxygen to the heart, and with the hallmarks of excessive reactive oxygen species (ROS) and inflammation accumulation, cardiomyocyte death, fibrosis and scar formation.4 NICM is characterised by inflammation, oxidative stress, hypertrophy or dilatation, fibrosis and cardiomyocyte death,1, 5 which aggregates cardiac dysfunction. NICM includes hypertrophy, septic and dilated cardiomyopathy (DCM), myocarditis (MC), chemotherapeutic cardiotoxicity, senescent cardiac dysfunction and cardiac allograft rejection1, 6 (Figure 1). Currently, NICM accounts for more than 50% of all heart transplantations and is considered the primary etiological factor for the progression to heart failure (HF).1, 5, 7, 8 Although NICM patients benefit from considerably improved management strategies, which markedly attenuate patients’ symptoms and prolongs patients’ survival,7, 8 the morbidity and mortality associated with NICM are still serious medical issues.7, 9, 10 Therefore, identifying novel biomarkers and therapeutic strategies for the early diagnosis, prevention and treatment of NICM and ultimately improving the prognosis of this disease has become an urgent need.
The inflammation is regarded as causal factor in the development of NICM and subsequent progression to HF.11, 12 The lasting inflammation will result in myocardial remodelling due to the infiltration of inflammatory cells and mediators into the ventricle.12, 13 Neutrophils serve as the most abundant leukocytes in the human circulation and the principal cell type, which is considered a crucial predictive indicator for inflammation once they are excessive and accumulate,12 and neutrophil hyperexpression is linked to the pathophysiological characteristics of patients with NICM and HF.14 In response to inflammatory conditions, neutrophils quickly arrive at the injury site and generate and release a large pool of ROS and bioactive lipid mediators, including myeloperoxidase, matrix metalloproteinase, neutrophil elastase, cathepsin G and cathelicidins, which not only evade detection by immune cells but also sustain the inflammatory response in tissues.12, 14 Recently, an increasing number of investigations have investigated the role of neutrophil-derived alarmins such as S100 calcium binding proteins A8 and A9 (S100A8/A9, also known as Calprotectin or MRP8/14) in regulating myocardial remodelling by mediating the systemic or local inflammatory response.12, 15
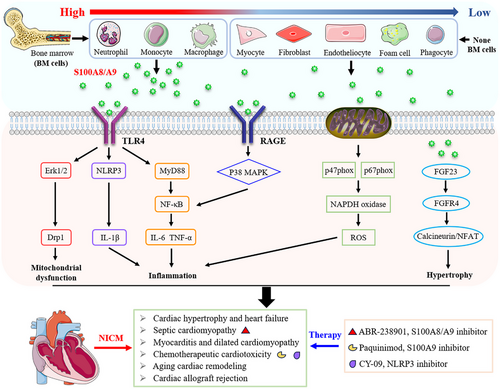
S100A8/A9 plays important roles in CVDs, including ischaemic cardiomyopathies and atherosclerosis, by exerting proinflammatory effects on cardiomyocytes, fibroblasts and endothelial cells. S100A8/A9 aggravates ischaemic HF by activating the receptors of advanced glycation endproducts (RAGE) and Toll-like receptor 4 (TLR4).11, 16 Furthermore, S100A8/A9 has been recognised as a predictor and potentially mediator for HF caused by acute myocardial infarction.17 This review explores the known functions of S100A8/A9 in biological systems, proposes pathways underlying its mechanisms of action, and examines its therapeutic potential. These findings highlight the role of S100A8/A9 in regulating inflammation, its involvement in NICM, and its effects on phagocyte migration and microtubule reorganisation. Finally, clinical studies on S100A8/A9 are discussed, aiming to inspire new approaches for NICM treatment.
2 OVERVIEW OF S100A8/A9 BIOLOGICAL CHARACTERISTICS
2.1 Genetic and structural specificity
S100A8/A9 is a member of the S100 protein family (including 25 members: S100A1‒S10016, S100G and S100B) and has a low molecular weight.18 This protein is encoded by the S100A8/A9 gene, which is located at a chromosomal region of 1q21 and an extended region of chromosome 3 in humans and mice, respectively.19 The S100A8 gene is indispensable during early embryonic development, and null mutations in this gene due to embryo resorption are lethal in mice.20 In contrast, homozygous S100A9 gene-deficient (S100A9−/−) mice are viable, fertile and phenotypically healthy.21 Interestingly, the myeloid and peripheral leukocytes from S100A9 null mice also no longer express the S100A8 protein, which is probably due to the stability deficiency of S100A8 in the absence of its heterodimer binding complex S100A9.22 In other words, S100A9−/− mice function similar to S100A8/A9 double-knockout mice.21, 22 Nevertheless, evidence has demonstrated that S100A9 gene knockout has no effect on the transcript level of S100A8.22 In addition, in bone marrow-specific S100A9-overexpressing mice, the S100A8 protein expression level is also upregulated.23
The protein molecular weights of S100A8 and S100A9 are 10.8 kDa (composed of 93 amino acids) and 13.2 kDa (composed of 113 amino acids), respectively.24 S100A8 and S100A9 proteins account for approximately 40% of the total cytoplasmic protein in myeloid neutrophils, whereas they account for only approximately 1% of total monocyte cytosolic proteins.25, 26 However, they are also widely expressed in epithelial cells and phagocytes,27 aortic endothelial cells,28-30 foam cells,31 keratinocytes,32 vessel smooth muscle cells,33 fibroblasts34 and macrophages35, 36 in response to proinflammatory stimuli, hyperglycaemia and increased levels of reactive oxygen intermediates. S100A8/A9 can be combined through various modes, including heterodimers, homodimers and tetramers.37-39 The structural stability of homodimers is weak; therefore, the S100A8 and S100A9 proteins form noncovalently linked heterodimer complexes (also referred to as calprotectins), which are the major structures that are present under physiological conditions and are known to possess diverse biological features.37, 40-42 S100A8/A9 heterodimers can form (S100A8/A9)2 tetramers once the concentration of calcium is appropriate, which is essential for both intracellular and extracellular activities in regulating autocrine and paracrine signalling pathways.38, 39
2.2 Secretion and biological roles of the S100A8/A9 protein
There are two kinds of secretory modes for the S100A8/A9 protein: active secretion and passive release from certain special cells. Extracellular S100A8/A9 has antimicrobial activity after it is released through active secretion.43-45 However, S100A8/A9 proteins have no identifiable signal peptide sequence, which is the classic indicator of protein secretion via the endoplasmic reticulum/Golgi.43 Nevertheless, it seems that the extracellular secretion of S100A8/A9 relies on ROS and ATP-sensitive potassium exchange.46 In contrast, the passive release of S100A8/A9 is usually dependent on neutrophil activation, leading to necrosis and the formation of neutrophil extracellular traps, which kill bacteria by forming extracellular fibers via granule protein release.47
The S100 protein family can exhibit both intracellular and extracellular biological functions on the basis of its exclusive molecular expression pattern. The intracellular neutrophil-derived S100A8/A9 protein complex was reported to positively regulate the activation of NADPH oxidase through the cytosolic phox proteins p67phox and Rac2, whereas the individual S100A8 protein is more likely to directly interact with p47phox.48, 49 Furthermore, the formation of (S100A8/A9)2 tetramers, which are based on calcium binding, plays a pivotal role in regulating tubulin polymerisation and microtubule reorganisation.39 Moreover, p38 mitogen-activated protein kinase (MAPK) specifically phosphorylates S100A9, which subsequently leads to the activation of Rac1 and Cdc42 and ultimately promotes microtubule formation and the transendothelial migration of phagocytes.50 In summary, the intracellular role of S100A8/A9 is primarily involved in regulating the migration of phagocytes.
Extracellular S100A8/A9 acts as both a potential active stimulator and regulatory mediator in response to various inflammatory conditions by activating the RAGE51, 52 and TLR4 signalling pathways.27, 53 In human primary macrophages, recombinant S100A9 promotes macrophage activation and induces the secretion of proinflammatory cytokines, including interleukin 1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α) and monocyte chemotactic protein 1 (MCP-1), through the activation of the nuclear factor kappa B (NF-κB), p38 MAPK and RAGE pathways, whereas it attenuates the levels of anti-inflammatory factors, including IL-10 and MRC1.54, 55 Data from comparative genome-wide bioinformatics analysis indicated that the TLR4/S100A8 axis could activate human monocytes and subsequently promote the expression of signalling pathways such as inflammatory cell activation and cell migration.56 In addition to its proinflammatory role, S100A8/A9 also serves as a regulator of the adaptive immune response. For example, S100A8/A9 can prevent excessive adaptive immune responses by suppressing the activation and antigen-presenting ability of dendritic cells.57
3 SIGNIFICANCE OF S100A8/A9 IN NONISCHEMIC CARDIOMYOPATHY
3.1 Hypertrophic myocardial remodelling and HF
Pathophysiology cardiac remodelling is characterised by myocardial hypertrophy, interstitial fibrosis and abnormalities of cardiac energetics and redox balance and ultimately leads to HF, which is closely related to the immunoinflammatory response and leukocyte infiltration.58, 59 Endogenous S100A8/A9 was reported to be a double-edged sword in regulating cardiac hypertrophy in response to various physiological stimuli.11 The expression of S100A8 is low in the physiological state, whereas it is significantly upregulated in neonatal rat cardiomyocytes (NRCMs) stimulated with thyroid hormone (TH).60 Excessive S100A8 results in cardiomyocyte hypertrophy by activating the myeloid differentiation factor-88 (MyD88) and NF-κB signalling pathways.60, 61 In addition, S100A8/A9 is overexpressed in phenylephrine-induced NRCMs through the activation of fibroblast growth factor 23 (FGF23) and calcineurin/nuclear factor of activated T cells (NFAT) signalling, which leads to cardiac myocyte hypertrophy.62 In contrast, removing the hypertrophic stimulus of norepinephrine results in the overexpression of both the mRNA and protein levels of S100A8/A9 in NRCMs.63 Moreover, the recombinant S100A8/A9 protein can protect against norepinephrine-induced cardiomyocyte hypertrophy by inhibiting calcineurin/NFATc3 pathway activation, whereas knockdown of S100A8/A9 abrogated these effects.63, 64 Additionally, S100A8/A9 contributes to cardiac injury and HF via overactivation of the β-adrenergic pathway. Activated S100A8/A9 aggravates the progression of cardiac fibrosis and HF by promoting fibroblast‒macrophage interactions, independent of inflammation, which is likely a key mechanism leading to increased collagen production.65 The mechanisms underlying the completely opposing functions of S100A8/A9 in regulating cardiac hypertrophy in response to different pathophysiological stimuli remains to be further investigated.
In preclinical studies involving aortic banding-induced pressure overload in mice, cardiac S100A8/9 levels were significantly increased, leading to cardiac hypertrophy and fibrosis. However, these prohypertrophic and profibrotic effects were largely alleviated by S100A9 deficiency or FGF receptor 4 (FGFR4) blockade.62 Furthermore, in an angiotensin II infusion-induced mouse cardiac remodelling model, researchers confirmed that angiotensin II increased S100A8/A9 expression in CD11b+Gr1+ neutrophils that infiltrated the heart, resulting in leukocyte infiltration, cytokine secretion, subsequent cardiac hypertrophy and fibrosis.66 Mechanically, the extracellularly released S100A8/A9 protein can activate RAGE, which is expressed on cardiac fibroblasts, and subsequently activate NF-κB signalling to further amplify proinflammatory cytokine products.66 In addition to the expression level of S100A8/A9, the nuclear localisation of S100A9 has also been reported to be associated with HF. Qi et al. reported that the IL-10 secreted by CD11b+Gr1+ myeloid-derived suppressor cells during exercise training promoted the translocation of phosphorylated STAT3 and S100A9 proteins to the nuclear compartment, which protected against isoproterenol-induced HF.67
3.2 Septic cardiomyopathy
Sepsis is characterised by an uncontrolled and systemic inflammatory response resulting from a dysregulated deleterious response to infection and frequently causes life-threatening multiple organ disorders.68, 69 The myocardial damage caused by sepsis is referred to as septic cardiomyopathy (SCM), which is characterised by acute and reversible myocardial structural and functional disorders and ultimately leads to high mortality.70, 71 S100A8/A9 was reported to play an important role in the pathogenesis of SCM.72, 73 Researchers reported that lipopolysaccharide (LPS) stimulation promoted S100A8/A9 mRNA expression in NRCMs, and this effect was abrogated by alamandine pretreatment, which is likely has a protective effect against SCM.74 In addition, a comprehensive bioinformatics analysis of microarray datasets of mouse and human heart tissue samples revealed S100A9 as one of the top ten hub genes, and the upregulation of S100A9 was further confirmed in myocardial cell lines of HL-1 (mouse) and AC16 (human) cells treated with LPS, which demonstrated that S100A9 was closely related to SCM.52, 75 Moreover, S100A8/A9 was found to be upregulated in cecal ligation and puncture (CLP)-induced mouse hearts36 and in LPS-stimulated mouse serum.52, 72 Similarly, the plasma S100A8/A9 levels in SCM patients were elevated.72
In a mouse model of endotoxemia, S100A9 gene deficiency or pharmacological blockade of S100A8/A9 with ABR-238901 exhibited a potential protective effect against the LPS-induced systemic inflammatory response and mitochondrial dysfunction in the myocardium.72 Moreover, specific myocardial overexpression of S100A8/A9 clearly aggravated cardiac disorders in LPS-treated mice, especially in terms of the ejection fraction of the heart, whereas S100A9 gene knockdown with small interfering RNA dramatically attenuated cardiac malfunction.52 Furthermore, elevated S100A8/A9 promoted CLP-induced mouse mortality, cardiac dysfunction, cardiomyocyte apoptosis, macrophage infiltration and superoxide production, and these effects were largely attenuated by S100A9 knockout or the administration of paquinimod, a unique inhibitor that prevents S100A9 from binding to the TLR4 receptor.36 Mechanistically, S100A8/A9 contributes to SCM and may activate TLR4‒ERK1/2-Drp1 signalling pathway-mediated mitochondrial fission and dysfunction.36
3.3 Myocarditis and dilated cardiomyopathy
MC is an inflammatory cardiac disorder that is triggered primarily by infections with various viruses, bacteria, protozoa or fungi in cardiac muscle tissue and most frequently leads to cardiac dysfunction and HF in young adults.76 S100A8/A9, which is secreted from myeloid and myocardial cells, has been reported to play an important role in the pathogenesis of viral and autoimmune MC. Fundamental research data from wild-type mice infected with coxsackievirus B3 (CVB3) and Pseudomonas aeruginosa confirmed that S100A8/A9 levels were significantly increased and that these proteins were highly expressed in infiltrated monocytes/macrophages and neutrophils of the heart and associated with the subsequent cardiac dysfunction.77, 78 Moreover, the elevated S100A8/A9-mediated inflammatory response and oxidative stress damage led to cardiac dysfunction, which was alleviated by S100A9 knockout.77 In addition, the MC occurred when S100A8 was exogenously applied to S100A9−/− mice infected with CVB3.77 Data from CVB3-infected HL-1 cells in vitro further verified the causal function of S100A8/A9 in promoting the production of ROS and increasing the number of CVB3 copies.77 However, the characteristics of the recombinant (R-) S100A8/A9 protein differ in that it clears circulatory cytokines rather than proinflammatory cytokines and exacerbates MCs. In a porcine cardiac myosin immune Lewis rat autoimmune myocarditis (EAM) model, R-S100A8/A9 (derived from Escherichia coli cells) application alleviated the severity of MC by decreasing the serum concentrations of proinflammatory cytokines, including IL-1β, IL-6 and TNF-α, and by inhibiting NF-κB expression.79 The possible anti-inflammatory function of S100A8/A9 may involve binding to and trapping proinflammatory, whereas the precise mechanism has yet to be explored. Data from the endomyocardial biopsies of clinical patients with CVB3 infection-associated MC indicated that the S100A8/A9 mRNA level was elevated, whereas this increase was not apparent in MC patients without CVB3 virus infection.77
On the basis of the histological diagnostic standard, MC is classified into different types according to the infiltration of eosinophilic, polymorphic and lymphocytic cells.80 Lymphocytic MC consistently progresses to inflammatory DCM.80 In MC patients with DCM, serum S100A8/A9 levels are only slightly increased, whereas this reflects myocardial inflammatory disease activity and is independent of virus infection.81 In addition, another clinical study involving serum proteomic analysis confirmed that the levels of circulating S100A8/A9, S100A4 and S100A12 were substantially elevated in patients with DCM.82 Additionally, the combination of these three proteins was superior to the individual indicators for diagnosing DCM.82 Therefore, elevated serum S100A8/A9 levels have been identified as promising diagnostic and therapeutic markers in DCM patients.
3.4 Chemotherapeutic cardiotoxicity
Chemotherapeutic agents that are used in cancer patients, such as doxorubicin (DOX, a broad-spectrum anthracycline agent) and ponatinib (tyrosine kinase inhibitor specifically used to treat chronic myelogenous leukaemia), result in irreversible cardiac injury and life-threatening cardiomyopathy, which increases the risk for congestive HF. Recently, S100A8/A9 was reported to be associated with the pathogenesis of chemotherapeutic agent-induced cardiotoxicity and injury. In a DOX-induced cardiotoxic model in C57BL/6J mice, elevated S100A8/A9 expression mediated the infiltration of neutrophils into the heart and subsequently led to cardiac dysfunction, myocardial injury and apoptosis.83 Low-intensity pulsed ultrasound therapy or S100A8/A9 inhibition by ABR-238901 treatment protected against DOX-induced cardiotoxicity by inhibiting S100A8/A9.83 Moreover, a microarray analysis of the hearts of type 2 diabetic mice revealed that S100A8 and S100A9 were exclusively expressed in mice with DOX-induced cardiomyopathy, inducing cardiac remodelling, the inflammatory response, oxidative stress and metabolism disruption.84 Elevated S100A8/A9 levels were related to p38 MAPK signalling activation and NF-κB-mediated IL-6 expression.84 Furthermore, DOX-induced cardiac S100A8/A9 overexpression was attenuated by organic cation transporter 3 (SLC22A3) knockdown through the RAGE/TLR4 signalling pathway, and S100A8/A9 gene deficiency protected against DOX-induced acute challenge, as reflected by cardiac malfunction and troponin I accumulation.85 In addition, ponatinib treatment increased S100A8/A9 levels in mouse hearts and subsequently caused myocardial and systemic inflammation by activating the cardiac and systemic myeloid TLR4‒NLRP3‒IL-1β signalling pathways, which was abolished by the administration of CY-09 (a specific NLRP3 inhibitor) or paquinimod (an S100A9 inhibitor).73
3.5 Ageing-associated cardiac remodelling
Ageing is recognised as one of the most crucial cardiovascular risk factors86 and is commonly characterised by cardiac diastolic dysfunction and hypertrophic remodelling.87 Senescent, inflammatory and fibrotic cardiac microenvironments are important predisposing factors for ageing-associated cardiomyopathy.88 The S100A8/A9-mediated inflammatory response has been reported to be involved in ageing-related cardiac remodelling. In a senescent model of male C57BL/6 mice, accumulated granulocytic myeloid-derived suppressor cells promoted the release of S100A8 and S100A9 into ageing hearts, which subsequently resulted in inflammatory responses and elevated the expression of osteopontin (OPN) in fibroblasts; these pathological phenotypes collectively led to cardiac fibrosis and dysfunction.89 In contrast, neutralising S100A8 and S100A9 inhibited age-induced cardiac proinflammatory phenotypes, intracellular ROS production and OPN overexpression in fibroblasts.89 In addition, RNA sequencing data from older adults have demonstrated that the S100A8/A9 level is increased in stem/progenitor cells (HSPCs) from peripheral blood and bone marrow and that elevated S100A8/A9 levels in individuals are associated with increased frailty, both of which are predicable for poor cardiovascular outcomes.90 Furthermore, other clinical data from apparently healthy middle-aged (aged 63‒68 years) individuals demonstrated that the concentration of plasma S100A8/A9 and the number of circulating neutrophils were positively associated with the incidence of coronary events and cardiovascular death.37
3.6 Cardiac allograft rejection
In addition to serving as a proinflammatory mediator, S100A8/A9 also functions as a blood marker for monitoring cardiac and renal allograft rejection during the early posttransplantation phase, and this dimeric complex has been reported to be involved in the pathogenesis of allograft rejection.91, 92 In a mouse vascularised heterotopic cardiac transplantation model, S100A9 inhibited antigen presentation by dendritic cells and subsequent T-cell priming by regulating the expression of the costimulatory molecules CD80 and CD86, which suppressed overactivation of the adaptive immune system.93 Moreover, S100A9 deficiency significantly promoted T-cell activation and aggravated cardiac allograft rejection.93 Cardiac allograft vasculopathy (CAV) is the primary factor of cardiac transplantation-induced graft loss and death. Whole-genome peripheral blood mononuclear cell microarray analysis and subsequent RT‒PCR analysis of samples from patients with CAV revealed S100A9 as the differentially expressed gene.94 These data support a previously unidentified function of S100A9 in immune cell biology.
4 CONCLUSION
Recently, great advancements have been made in the investigation of S100A8, S100A9 or S100A8/A9. Progress in technology has resulted not only in a comprehensive understanding of the structural specificity of S100A8 and S100A9 but also in a more accurate understanding of their biological roles. There is no doubt that S100A8/A9 plays an intracellular role in the regulation of phagocyte migration and microtubule reorganisation, as well as extracellular roles in the regulation of various inflammatory conditions by activating multiple signalling pathways, such as the classical RAGE and TLR4 pathways (Figure 2). The significance of S100A8/A9 in NICMs has become an increasingly intriguing research topic. Many studies have confirmed its strong association with the pathogenesis of NICMs. Elevated circulating or cardiac S100A8/A9 concentrations are apparently related to inflammation and have become a primary prognostic marker for poor prognosis in patients with NICMs. However, the specific regulatory mechanism and pathway of S100A8/A9 has yet to be explored because of the various biological roles associated with intricate transcriptional and posttranslational modifications. In addition, numerous significant NICMs still need to be further studied, which highlights the need to explore the potential of S100A8/A9 in the diagnosis and treatment of NICMs in the future.
AUTHOR CONTRIBUTIONS
Qiu-Yue Lin searched and reviewed the literature. Qiu-Yue Lin, Wen-Xi Jiang and Hui-Hua Li and drafted the manuscript.
ACKNOWLEDGEMENTS
This research work was supported by the Excellent youth science and technology talent project of Dalian (2024RY005) to Qiu-Yue Lin.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.




