Risk of gastric cancer in autoimmune gastritis and pernicious anaemia: Insights from Mendelian randomization and multi-omics analysis
Abstract
Background
The newly onset debate surrounding the risk of gastric cancer (GC) in autoimmune gastritis (AIG) and pernicious anaemia has intensified. It is necessary to supplement higher level research evidences to settle this issue.
Methods
Two-sample Mendelian randomization (MR) analysis using inverse variance weighted method was conducted to reveal the causal relationship between pernicious anaemia and GC. Because of the absence of available summary statistics for AIG at present, we used pernicious anaemia as a proxy exposure, as it was frequently used interchangeably. The multi-omics characteristics of AIG and pernicious anaemia were further explored through proteome-wide MR, colocalization, and transcriptome sequencing analysis.
Results
MR analysis found pernicious anaemia was causally associated with a higher risk of GC (odds ratio: 1.16, 95% confidence interval [1.03, 1.31], p = .018). Sensitivity analyses confirmed the stability of the results. The up-regulation of genes involved in gastric dysplasia and carcinogenesis, including receptor activity-modifying protein 3, fibroblast growth factor 3, transforming growth factor beta-2 and tumour-associated calcium signal transducer 2, suggested potential mechanisms underlying the risk of GC in AIG.
Conclusions
These results emphasized the independent link from AIG and pernicious anaemia to GC. Therefore, endoscopy follow-up for GC screening in AIG is still appealed.
1 BACKGROUND
Autoimmune gastritis (AIG) is a chronic inflammatory disorder characterized by autoimmune-mediated atrophy primarily affecting the oxyntic gastric mucosa, particularly the fundus and corpus of the stomach. This is in contrast to chronic gastritis caused by Helicobacter pylori (H. pylori) infection, which predominantly targets the antral mucosa.1 The disease course of AIG is usually long, with progressive atrophy of the oxyntic mucosa leading to achlorhydria and abnormal absorption of vitamin B12, and eventually, resulting in pernicious anaemia (PA), the end-stage manifestation form of AIG,2 which has been used as a synonym for AIG for decades.1 Given its insidious onset and poor prognosis, AIG/PA has garnered increasing awareness in contemporary medical practice.
For decades, AIG/PA has been widely regarded as a risk factor for gastric cancer (GC).1, 3 However, recent studies have raised doubts about this conventional concept. Two prospective single-arm cohort studies have suggested that AIG may merely be a risk factor for gastric neuroendocrine tumours (NET) rather than GC, implying that AIG/PA could be a relatively benign condition.4, 5 While the association between AIG/PA and GC may be confounded by the coincidence of H. pylori infection.6 Nevertheless, it is important to note that these studies have their own limitations, such as a relatively short follow-up duration for assessing carcinogenesis and a lack of sensitive endoscopic surveillance for detecting early stage GC. Extrapolating the findings may potentially lead to adverse consequences in terms of GC screening.7, 8
Given the ongoing debate surrounding the causal relationship between AIG/PA and GC, it is urgent to supplement higher level research evidence to shed more light on this issue. Mendelian randomization (MR) is designed to elucidate causal relationships between exposures and outcomes by leveraging inherited single nucleotide polymorphisms (SNPs), thus minimizing bias due to potential confounders.9 MR represents an ideal approach for investigating the direct causal link between AIG/PA and GC, independent of confounding from H. pylori infection. Therefore, we conducted comprehensive MR analyses to ascertain whether genetically determined AIG/PA independently leads to an increased risk of GC. Given the absence of available Genome-Wide Association Studies (GWAS) data for AIG at present, we opted to use PA as a proxy exposure, as PA is considered the end-stage manifestation of the AIG spectrum and is often used interchangeably with AIG in epidemiological studies.1-3, 8 We further performed MR analyses to replicate the causal associations between PA and gastric atrophy, gastric benign neoplasm, gastric NETs and thyroid cancer,4 in order to validate the use of PA as the surrogate exposure. Moreover, we employed proteome-wide MR and colocalization analyses to explore potential molecular mechanisms underlying the carcinogenesis of PA, which were subsequently confirmed by transcriptome sequencing data of AIG.
2 METHODS
2.1 Mendelian randomization
2.1.1 Data sources
The series of two-sample MR analyses were based on publicly available summary statistics of GWAS mainly from FinnGen, a large-scale genomics initiative analysing over 500 000 Finnish biobank samples and correlated genetic variation.10
For PA, 3694 cases were included from FinnGen (ICD-10: D51). In addition, 180, 2901, 118, 1907 and 1423 cases were included for atrophic gastritis,11 gastric benign neoplasm, gastric NET, thyroid cancer and GC10 (Table S1). According to the description from FinnGen, there was a slight overlap between PA and GC cohorts, with a Jaccard index of 9.81%. While under this circumstance, estimated bias due to sample overlap did not increase Type-1 error rate (Supporting Information section 1).12 Overlaps between PA and gastric benign neoplasm/gastric NET/thyroid cancer were also quantified by Jaccard index of 3.04%, 1.66% and 0.29%, respectively.
For the proteome-wide MR analyses, summary statistics from a large-scale protein quantitative trait loci study of genetic associations with levels of 4907 proteins from 35 559 Icelandic participants13 were used to explore the molecular carcinogenesis mechanisms underlying PA.
2.2 Selection for instrumental variables
MR analyses were based on three core assumptions: (I) Genetic variants are robustly associated with exposure, that is, PA (relevance); (II) genetic variants proxying exposure must be independent from H. pylori infection and other confounding factors (independence) and (III) genetic variants proxying exposure can only affect outcomes only through exposure itself (exclusion restriction). SNPs strongly associated with exposure, that is, PA, were selected as instrumental variables (IVs) based on the following criteria. (i) SNPs were genome-widely significant (p < 5 × 10−8); (ii) SNPs were clumped with a 10 000 kb window and linkage disequilibrium (LD) threshold of r2 < .001; (iii) excluding palindromic SNPs; (iv) excluding SNPs with F statistic <10 to avoid the impact from weak IVs; (v) excluding SNPs through Steiger test to avoid reverse causality inference and (vi) excluding SNPs associated with known confounders, including H. pylori infection, through screening PhenoScanner14 and GWAS Catalog.15 F statistic is defined as beta2/Se2, and R2 is the proportion of variance explained by the IVs, which was calculated by 2 × beta2 × EAF × (1-EAF), where beta represents the effect size of the IVs in GWAS, Se represents the standard error, and EAF represents the effect allele frequency.
2.3 Statistical analysis
As main analysis, inverse variance weighted (IVW) method was performed. Cochrane's Q was used to indicate heterogeneity. Fixed effect model was applied for IVW method analysis, when there was a significant heterogeneity (I2 > 50%), the random effect model would be adopted.
Apart from IVW method, various sensitivity analyses were combined, including MR-Egger regression, Bayesian weighted MR (BWMR),16 constrained maximum likelihood and model averaging (cML-MA),17 MR-PRESSO,18 and weighted median (WM)19 methods to avoid potential assumption violations and possible biases in MR analyses to strengthen the causal relationships between PA and associated conditions. For proteome-wide MR analyses,20 IVW, MR-Egger regression, BWMR, cML-MA and WM methods were combined. For proteins causally associated with PA, KEGG enrichment was further analysed to reveal the potential mechanisms underlying PA.
2.4 Colocalization analysis
Colocalization analysis provided insights into the connection between expression levels (trait 1) and PA (trait 2). The Bayesian method was used to assess the evidence for the following five exclusive hypotheses: (H0) no link to either trait; (H1) linked only to trait 1; (H2) linked only to trait 2; (H3) both traits are linked, but with separate causal variants and (H4) both traits are linked, and with the same shared causal variant. Probabilities for each hypothesis were calculated. A posterior probability of 0.7 or above for a shared causal variant (H4) was deemed strong evidence of colocalization.
2.5 Transcriptome sequencing analysis
For additional verification of molecular mechanisms underlying PA revealed by proteome-wide MR in European population, transcriptome sequencing (Series GSE233973)21 data from Asia cohort recruiting 14 AIG patients were used to enhance the generalizability of the findings. The gene expression levels of proteins involved in carcinogenesis of AIG/PA revealed by proteome-wide MR were verified.
3 RESULTS
3.1 Causal relationship between PA and GC
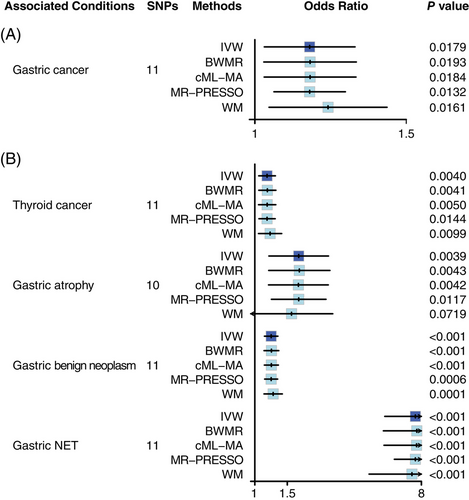
MR analyses revealed the causal link from genetically proxied PA, that is, end stage form of AIG, to GC (Figure 1A, Table S2). According to IVW method, PA was causally associated with a higher risk of GC (odds ratio [OR]: 1.16, 95% confidence interval [CI] [1.03, 1.31], p = .0179), and leave-one-out analysis indicated a stable result (Figure S1). In sensitivity analyses, BWMR method (OR: 1.16, [1.02, 1.31], p = .0193), cML-MA method (OR: 1.16, 95% CI [1.03, 1.31], p = .0184), MR-PRESSO method (OR: 1.16, [1.05, 1.27], p = .0132) and WM method (OR: 1.22, 95% CI [1.04, 1.43], p = .0161) simultaneously supported the result of IVW method analysis. Cochran's Q statistic indicated absent heterogeneity (pheterogeneity = .7981). MR-Egger intercept method indicated absent pleiotropy (ppleiotropy = .7586). MR-PRESSO found no outliers affecting the result. Browsing PhenoScanner and GWAS Catalog confirmed that all the SNP variants proxying PA (Table S3) are not related to H. pylori infection.
3.2 Causal relationships between PA and AIG-related conditions
As positive reference, the known relationships from AIG to thyroid cancer, gastric atrophy, gastric benign neoplasm and gastric NET were also verified through MR analyses (Figure 1B, Table S4). In IVW method analyses, PA was causally associated with higher risk of thyroid cancer (OR: 1.17, [1.05, 1.29], p = .0040), gastric atrophy (OR: 1.73, [1.19, 2.51], p = .0039), gastric benign neoplasm (OR: 1.23, [1.13, 1.33], p < .0001) and gastric NET (OR: 7.42, [5.07, 10.88], p < .0001). Sensitivity analyses, including BWMR, cML-MA, MR-PRESSO and WM methods, also simultaneously supported the results of IVW method analyses, and there was no evidence of heterogeneity or pleiotropy. MR-PRESSO found no outliers affecting the result.
3.3 Exploration of molecular carcinogenesis mechanisms underlying AIG and PA
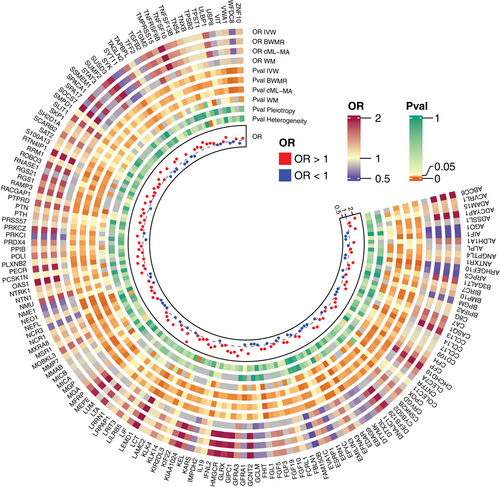
The causal associations between 4907 protein expression levels and PA were examined via proteome-wide MR analysis to reveal the molecular mechanism underlying PA. Through IVW method analyses and sensitivity analyses, including BWMR, cML-MA and WM method analyses, 158 protein expression levels were identified to be causally related to PA (Figure 2 and Table S5), including 54 down-regulated proteins and 104 up-regulated proteins (Figure 3A). KEGG enrichment (Figure 3B) accordingly focused on cytokine–cytokine receptor interaction (ACVRL1, BMP10, CCL13, CCL14, CCL17, CNTFR, EDAR, IFNL2, IL19, LIF, LTA, transforming growth factor beta-2 [TGFB2], TNFRSF6B, TNFSF10 and TNFSF13B), natural killer cell mediated cytotoxicity (KIR2DL1, MICA, MICB, NCR1, NCR3, SH2D1A, SYK, TNFSF10 and ULBP1) and PI3K–AKT signalling pathway (EFNA3, fibroblast growth factor 10 [FGF10], FGF19, FGF3, FGF5, LAMC2, NTRK1, SYK and TNXB). Intriguingly, GC signalling pathway was enriched (FGF10, FGF19, FGF3, FGF5 and TGFB2). Other pathways, including RAP1 signalling pathway, axon guidance, viral protein interaction with cytokine and cytokine receptor, regulation of actin cytoskeleton, NF-κB signalling pathway, JAK-STAT signalling pathway, biosynthesis of cofactors, purine metabolism and nucleotide metabolism pathways, were also involved. Among these proteins, receptor activity-modifying protein 3 (RAMP3) participates in gastric metaplasia response to severe injury, and colocalization analysis observed a tight relationship between RAMP3 and PA (Figure 3C), with a probability of 71% to share a single variant (rs1294873).
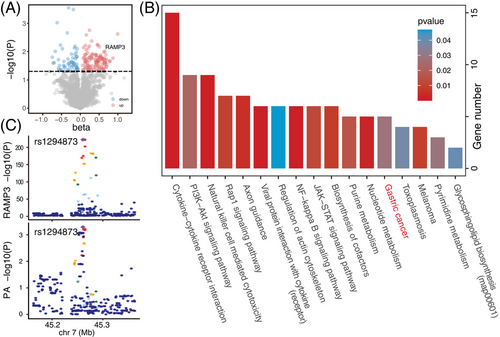
Transcriptome data from an Asian AIG dataset were also analysed, and the diagnosis of ‘pure AIG’ was verified by positive anti-parietal cell antibody and negative anti-H. pylori antibody. Through principal coordinate analysis, difference between mucosa transcriptome patterns of AIG and H. pylori infection was depicted (p = .0369), which suggests that the transcriptomic alterations associated with AIG cannot be attributed solely to comorbid H. pylori infection (Figure 4A). Moreover, the gene expression levels of several proteins unveiled by proteome-wide MR were further verified in Asian AIG, including up-regulated RAMP3, FGF3 and TGFB2 (Figure 4B). The up-regulated tumour-associated calcium signal transducer 2 (TACSTD2) (Figure 4C) reflects the transition from metaplasia to dysplasia, also hinting at the carcinogenicity of AIG.

4 DISCUSSION
In this study, we utilized MR to elucidate the causal relationship from genetically proxied AIG, using PA as a surrogate exposure, to GC. Our findings demonstrated a direct link between AIG/PA and an increased risk of GC, supported by positive methodological references with various AIG-related conditions, including gastric atrophy, gastric benign neoplasm, gastric NET, and thyroid cancer. These results further underscore the validity of our approach and the robustness of our conclusions. In addition, we conducted multi-omics analyses of PA and AIG in both European and Asian populations. KEGG enrichment analysis highlighted key signalling pathways such as cytokine–cytokine receptor interaction, natural killer cell mediated cytotoxicity and PI3K–AKT signalling, with a particular emphasis on the GC pathway. Furthermore, the up-regulation of RAMP3, FGF3, TGFB2 and TACSTD2 underscored the potential carcinogenic properties of AIG, providing valuable insights into the molecular mechanisms underlying the development of GC.
Currently, there is an ongoing debate regarding the risk of GC in ‘pure’ AIG patients without H. pylori infection. A single-arm cohort study involving 211 H. pylori-negative AIG patients found no incident cases of invasive gastric malignancies over a mean follow-up period of 7.5 years, leading to the conclusion that the corpus atrophy characteristic of AIG was not linked to an increased risk of GC.4 However, other observational cohorts and animal studies have provided evidence supporting the potential carcinogenicity of AIG.7, 22 Our study's findings align with the latter perspective, suggesting that AIG/PA may indeed be a risk factor for GC, especially in its end-stage form. Although H. pylori infection was not considered in FinnGen database, we have also screened the human GWAS Catalog and PhenoScanner. Because no record indicated the potential relationship between SNPs proxying PA and confounders, including H. pylori infection, the confounding of H. pylori infection was ruled out through MR analysis. Nevertheless, our results do not discount the conclusions of previous single-arm cohorts.4, 5 The disparity in results between studies may be attributed to differences in the stages of AIG disease. In our study, the exposure of interest was PA, which represents the advanced stage of AIG, whereas participants in observational studies often presented with moderate atrophy in the oxyntic mucosa. By synthesizing the outcomes of existing studies, we question the characterization of AIG as a benign condition. The recommendation for endoscopy follow-up for GC screening in AIG should remain, but with the possibility of individualizing the examination frequency based on the stage of the disease.
The implementation of two-sample MR necessitates GWAS summary statistics for both exposure and outcome. However, due to the challenges associated with confirming the diagnosis of early AIG, stemming from the instability of AIG's autoantibodies and atypical endoscopic features, comprehensive GWAS data covering the entire disease spectrum of AIG is currently unavailable.1, 22 As an autoimmune-mediated condition, AIG is characterized by the presence of positive autoantibodies, including anti-parietal cell antibodies and anti-intrinsic factor antibodies, as well as hypergastrinemia, which leads to enterochromaffin-like cell hyperplasia. The atrophy of oxyntic-secreting mucosa results in impaired absorption of vitamin B12, culminating in PA.1 PA serves as the end-stage manifestation of AIG and has been interchangeably used with AIG in prior research studies.23, 24 Consequently, GWAS summary statistics for PA were used as a proxy for AIG in our MR analyses. To validate the appropriateness of using PA as a surrogate for AIG, we examined the relationships between PA and other AIG-related conditions, such as gastric atrophy, gastric benign neoplasms, gastric NETs and thyroid cancer, as previously established in the literature.4, 5, 7 These positive associations served as confirmation that utilizing PA to represent AIG in our analyses was a justifiable and reasonable approach.
The molecular mechanisms behind the carcinogenic potential of AIG were explored through proteome-wide MR and colocalization analyses. Key pathways implicated in immune response and carcinogenesis, including cytokine–cytokine receptor interaction, natural killer cell mediated cytotoxicity and PI3K–AKT signalling pathway.25, 26 Particularly noteworthy was the enrichment of GC signalling pathway, providing further evidence of the carcinogenic capacity of AIG. Differences in transcriptome sequencing data between gastric mucosa of AIG and H. pylori-infected gastritis were observed. In the Japanese cohort, none of the participants used gastric acid suppressants or had a history of gastrectomy. Diagnosed AIG cases were confirmed to be negative for anti-H. pylori IgG, ruling out comorbid H. pylori infection.21, 27 These stringent criteria ensured the diagnosis of ‘pure AIG’, consistent with the previous single-arm study.4 Up-regulations of RAMP3, FGF3 and TGFB2 were confirmed in this Asian cohort. RAMP3 was known to be involved in the gastric metaplasia response to severe injury,28 whereas FGF3 and TGFB2 played critical roles in the GC signalling pathway. TACSTD2 was indicative of the transition from metaplasia to dysplasia,29, 30 collectively suggesting the potential carcinogenicity of AIG.
This study has certain limitations that should be acknowledged. First, although the use of PA, which is by definition involving autoimmune mechanism, as a surrogate exposure was deemed reasonable, it is important to note that our results may not fully encapsulate the entire spectrum of AIG. Though detailed exclusion criteria for H. pylori infection are not publicly available in FinnGen's metadata, the requirement for immune dysfunction in PA classification strongly suggests that these cases predominantly reflect autoimmune aetiology rather than secondary causes of vitamin B12 deficiency, including multifocal atrophic gastritis or H. pylori infection. Importantly, 31% (1145/3694) of PA cases in FinnGen underwent confirmatory gastric biopsies, further supporting diagnostic rigour. It is essential for future studies to validate our findings once the GWAS summary statistics of AIG become available. Second, the inability to conduct MR analyses based on Asian populations due to the lack of GWAS data presented a notable limitation. Moreover, the current GWAS data do not allow for the classification of GC according to location and histopathology,31 necessitating further investigations to enhance the depth of our current findings. Besides, beyond the risk of GC in ‘pure’ AIG, it is crucial to consider that AIG concomitant with H. pylori infection may introduce an additional risk of GC.6 Our study demonstrated that the causal relationship between AIG and GC remains independent of confounding H. pylori infection. Future studies may benefit from employing multivariable MR analyses to address the interaction between AIG and H. pylori infection.
5 CONCLUSION
In conclusion, this study emphasized the independent link from AIG to GC. Therefore, AIG might not be labelled as a relatively benign lesion with no risk of GC, and endoscopy follow-up for GC screening in AIG is still appealed.
AUTHOR CONTRIBUTIONS
Shengan Zhang, Liang Dai and Guang Ji conceptualized this study. Shengan Zhang, Ziqi Zhang and Wenjun Zhou were involved in the analyses and manuscript drafting of this study. Ziqi Zhang, Liang Dai, Guang Ji and Shengan Zhang were involved in methodology and results interpretation. Yanqi Dang revised the manuscript. Shengan Zhang was involved in the visualization of the results. Wendong Huang provided critical revision of the manuscript.
ACKNOWLEDGEMENTS
We appreciate Professor Jeffrey S. Isenberg M.D., M.P.H. (from Arthur Riggs Diabetes & Metabolism Research Institute, City of Hope National Medical Center, Duarte, California, USA) for his constructive suggestions. Moreover, we would like to acknowledge all the researchers who shared GWAS and multi-omics data. This work was supported by National Natural Science Foundation of China: 82320108022
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable. Included studies and databases had been approved by corresponding ethical review committees, respectively.
Open Research
DATA AVAILABILITY STATEMENT
Publicly available summary statistics of GWAS from FinnGen (www.finngen.fi/en) can be obtained by submitting a data request proposal. GWAS summary statistics of Gastric atrophy (GCST90044136) can be downloaded from GWAS Catalog (www.ebi.ac.uk/gwas/). Summary data of 4907 protein levels were stored in deCODE (www.decode.com/summarydata/). Transcriptome sequencing data (Series GSE233973) were obtained from Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/).




