Post-COVID-19 pulmonary fibrosis: Mechanisms, biomarkers, and therapeutic perspectives
Abstract
Post-COVID-19 pulmonary fibrosis (post-CPF) has emerged as a serious complication with profound implications for long-term respiratory health. This short review explores the multifactorial mechanisms underlying post-CPF, emphasising the role of oxidative stress, epithelial-to-mesenchymal transition (EMT), and dysregulated immune responses. Key signalling pathways, such as TGF-β, WNT, and Cadherin, are pivotal in fibrosis progression, offering potential therapeutic targets. Biomarkers, such as MUC4, KRT5, and ATP12A show promise for early detection and therapeutic targeting, as they share molecular features with idiopathic pulmonary fibrosis (IPF) and fibrotic interstitial lung diseases (f-ILDs), suggesting opportunities to repurpose antifibrotic therapies. Despite these advancements, significant gaps remain in understanding the cellular and molecular mechanisms underlying fibrosis progression, hindering effective management of post-CPF. Addressing these challenges through a targeted approach is critical to improving outcomes for survivors of severe COVID-19.
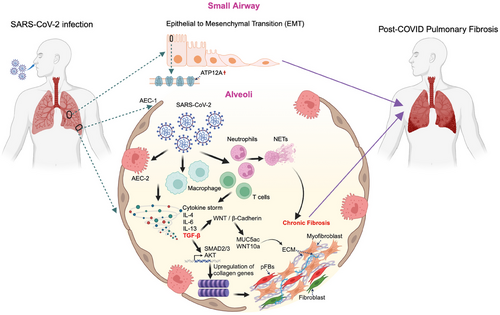
Pulmonary fibrosis (PF) is a severe, progressive condition marked by excessive collagen deposition in the interstitial lung tissue, often leading to compromised lung architecture and function. This chronic disease is frequently fatal and poses a significant challenges in management. In the acute phase of the disease, PF has been reported in several patients, with some requiring immediate lung transplantation.1 Notably, lung cryo-biopsies from critically ill patients or post-mortem revealed substantial collagen deposition and impaired lung aeration, which was correlated with features of fibrosis observed shortly before biopsy using high-resolution computed tomography (HRCT).1 Furthermore, a multicentre study reviewing explanted lungs in COVID-19 patients revealed diffuse alveolar damage (DAD).2 Over time, DAD progressed to organised fibrosis characterised by bronchiolisation, microscopic honeycombing, and alveolar architectural distortion—hallmarks of advanced fibrosis.2 By tracking CT changes in the lung parenchyma, a progression from early ground-glass opacities (GGO) to fibrotic patterns, including reticulation, bronchial distortion, and honeycombing was observed in a recent study by Gulati and Lakhani.3 This evolution was documented within a month, underscoring the rapidity with which COVID-19 can trigger fibrotic processes in susceptible patients.3
Elevated levels of transforming growth factor-β (TGF-β), a central mediator of fibrosis, have been consistently observed in COVID-19 patients.4 Severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection damages alveolar epithelial type 2 (AEC2) cells, triggering oxidative stress and an innate immune response characterized by macrophage accumulation.5 Both oxidative stress and infiltrating macrophages enhance TGF-β production, which, in turn, stimulates the secretion of cytokines such as interleukin (IL)-4, IL-6, and IL-13, driving the development and progression of PF.6 Additionally, TGF-β activates downstream signalling pathways, such as the AKT pathway, which promotes the expression of fibrotic markers like β-catenin and collagen type II alpha 1 chain (COL2A1).7
Emerging evidence highlights the involvement of the epithelial-to-mesenchymal transition (EMT) as a critical process in SARS-CoV-2-associated PF.5, 8, 9 Using single-cell RNA profiling several mesenchymal state-related genes were identified, and shared between COVID-19 and idiopathic pulmonary fibrosis (IPF) patients.10 SARS-CoV-2 infection induces several EMT-related genes (e.g., ZEB1, Slug, AXL, vimentin)11, 12. Additionally, COVID-19 patients have demonstrated downregulation of a transcription factor Nuclear factor erythroid 2-related factor 2 (Nrf2)-associated genes that protect against PF, suggesting its potential role in mitigating COVID-19-induced PF.13 These findings underscore the intricate mechanisms driving EMT and highlight the need of potential therapeutic targets for managing SARS-CoV-2-induced PF.
Recently, Meis et al. and Kooistra et al. provided complementary insights into the molecular mechanisms underlying PF in severe COVID-19. Using 33 autopsy samples, they have identified significant fibrotic responses characterised by the upregulation of genes such as COL1A2, COL3A1, COL6A3, and LOX, which are associated with collagen biosynthesis, extracellular matrix (ECM) remodelling and fibroblast activation.14 These pathways closely resemble those seen in IPF, suggesting shared mechanisms of fibrosis in both diseases.14 Additionally, pathological fibroblasts (pFBs) expressing markers like CTHRC1 and SPARC were found to be enriched in both COVID-19-related fibrosis and IPF, suggesting a common fibrotic process and supporting the potential for repurposing antifibrotic therapies approved for IPF in the treatment of COVID-19 fibrosis.14 Complementing these findings, Kooistra et al. focused on critically ill patients and revealed the involvement of inflammation and coagulation pathways, with genes like PADI4, MMP8, and PDE4D contributing to fibrosis progression.15
Long-term fibrotic outcomes have also been well-documented in survivors of severe COVID-19.16 A study by Huang et al. reported persistent radiological abnormalities in 32 out of 39 patients 6 months post-infection, particularly amongst those requiring advanced respiratory support during the acute phase. Although improvements were noted at the 1-year follow-up, however, patients with severe disease demonstrated a higher likelihood of progressing to irreversible fibrosis. These radiological changes were often accompanied by functional impairments, with significant reductions in total lung capacity (TLC) and diffusion capacity for carbon monoxide (DLCO) persisting for up to a year.17 However, another study using surgical lung biopsies from patients with unresolved symptoms months after COVID-19 infection substantiated the presence of post-COVID-19 pulmonary fibrosis (post-CPF).18 Whilst several studies have focused on the clinical manifestations and radiological features of post-CPF, there is a paucity of data on the cellular components and molecular pathways driving this fibrotic response. This knowledge gap hinders the development of targeted therapies for managing or preventing fibrosis in post-COVID-19 patients.
Recent studies have focused on addressing some of the knowledge gaps mentioned above. In one study, Batah et al. provided crucial molecular insights into the pathogenesis of post-CPF, revealing shared mechanisms with fibrotic interstitial lung diseases (f-ILDs).19 The study described the pathological progression from subacute COVID-19, known as the organising diffuse alveolar damage (ODAD) phase, to chronic PF in certain patients. One key finding was the identification of 31 genes commonly expressed across ODAD, post-CPF, and f-ILDs, with MUC4 and KRT5 as probable early-stage biomarkers. These genes are believed to play a pivotal role in the transition from the acute fibrotic phase (ODAD) to the more advanced chronic fibrotic phase (post-CPF).19 Enrichment analyses further identified the upregulation of transcription factors SMAD2 and SMAD3, key regulators of the TGF-β signalling pathway, which is essential for driving epithelial–mesenchymal transition (EMT) and the progression of fibrotic diseases, including post-CPF.19 The study also revealed 86 genes commonly expressed in CPF and f-ILDs but absent in the ODAD group, suggesting their role as “fibrosis-related genes” that sustain chronic fibrosis. These upregulated genes were enriched in WNT and Cadherin signalling pathways, both of which are associated with fibrosis progression and EMT. Furthermore, the study confirmed higher expression of MUC5ac and WNT10a in CPF and f-ILDs, reinforcing their involvement in PF.19
Another study by Abdelgied et al. identified ATP12A overexpression as a key contributor to post-CPF. ATP12A, a nongastric H+, K+-ATPase, was found to be upregulated in the small airways of SARS-CoV-2-infected BALB/c mice and human lungs of post-COVID-19 fibrosis patients.19 This overexpression was associated with increased airway mucus accumulation, reduced airway surface liquid pH, and exacerbated fibrosis, mirroring patterns observed in IPF. Further, single-cell RNA sequencing confirmed elevated ATP12A expression, particularly in goblet cells, linking it to excessive mucin production and airway obstruction.20
The involvement of immune cells is likely significant in post-COVID-19 fibrosis. Notably, a study by Cheon et al. found that dysregulated respiratory CD8+ T cell composition or responses were associated with lung dysfunction in post-COVID-19 20. Single-cell transcriptomic analysis identified specific subsets of these CD8+ T cells, particularly CD69+CD103− and CXCR6hi effector-like tissue-resident cells, which exhibited heightened inflammatory and cytopathic features, potentially driving sustained tissue pathology.21 Neutrophils may also play a role, as increased neutrophil extracellular traps (NETs) have been detected in the serum of patients during the early post-acute phase.22 Additionally, Bingham et al. reported a significant reduction in circulating monocytes in late-resolving COVID-PF patients compared to early-resolving COVID-PF and healthy controls, which correlated with decreased lung function 22. Late-resolving COVID-PF patients also displayed upregulated MHC-II molecules on CD8+ T cells and downregulated expression on monocytes, a pattern similarly observed in IPF patients.23 These findings provide valuable insights into the immune mechanisms driving fibrosis and suggest potential avenues for therapeutic intervention.
1 CONCLUSION
PF as a complication of COVID-19 presents a significant clinical challenge with considerable implications for long-term patient outcomes. Current evidence highlights the multifactorial pathogenesis of post-COVID-19 PF, involving oxidative stress, EMT, dysregulated immune responses, and molecular pathways. Emerging biomarkers like MUC4, KRT5, and ATP12A provide opportunities for early diagnosis and targeted therapeutic interventions.
Additionally, the overlap in molecular mechanisms between COVID-19-induced fibrosis and IPF or f-ILDs underscores the potential for repurposing existing antifibrotic therapies. Despite these advances, significant gaps remain in understanding the cellular and molecular drivers of fibrosis progression. Bridging these knowledge gaps is essential for developing effective management strategies to improve outcomes for patients with post-COVID-19 PF (Figure 1)
AUTHOR CONTRIBUTIONS
UKS and SNB contributed to the preparation and collection of literature. UKS wrote and SNB edited the manuscript.
ETHICAL APPROVAL
Not applicable.




