Utilising single-cell sequencing in clinical nutraceutical research: Recent progress and perspectives
Abstract
Background
Single-cell sequencing technologies have revolutionised pharmaceutical research by providing in-depth insights into human biology at the single-cell level. These tools enable researchers to identify rare cell types and analyse cellular diversity within tissues, facilitating the discovery of new therapeutic targets and biomarkers. However, their application in nutraceutical research is still in its early stages.
Main Body
Unlike pharmaceuticals, which have well-defined chemical structures and mechanisms, nutraceuticals are food-based materials intended for specific medical purposes and often contain complex and diverse food chemicals. These molecules can work synergistically, producing multi-targeted effects in various tissues. Traditional bulk profiling methods for tissues and tumours do not adequately capture cellular heterogeneity or specific cellular responses to treatments. Therefore, advanced single-cell sequencing is crucial for dissecting tissues into distinct cell types, helping to clarify the underlying mechanisms at the cellular level. Many derivatives of functional foods have been marketed or assessed, demonstrating health benefits. However, mechanistic insights are lacking, with most current data derived from observational studies or traditional in vitro and in vivo models. Human clinical trials are needed to validate these nutraceutical effects and determine effective and safe dosages. Edible mushrooms have gained attention as nutraceuticals due to their medicinal properties. They have been observed to enhance immunity, reduce inflammation, and combat cancer. These effects have been attributed to their unique bioactive components and historical uses in traditional medicine. Epidemiological studies show that higher consumption of edible mushrooms is linked to a reduced risk of certain cancers.
Conclusion
In this review, we share important lessons learned in the design and execution of clinical trials focusing on white button mushrooms as anti-cancer nutraceuticals. We demonstrate the use of single-cell RNA sequencing (scRNA-seq) in nutraceutical research to capture the nuanced biological responses and health effects of dietary foods and their constituents.
1 INTRODUCTION
Single-cell RNA sequencing (scRNA-seq) has transformed pharmaceutical research by providing detailed and ‘non-biased’ insights into human responses—regarding both safety and effectiveness—at the cellular level.1 A significant challenge in treating diseases is the variability in drug responses amongst patients, which arises from cell-to-cell heterogeneity.2 scRNA-seq is valuable for understanding the heterogeneous complexities of diseases such as cancer, autoimmune diseases, metabolic disorders and neuro-degenerative conditions.3 Major organs and tissues, including the brain, lungs, liver, immune system,4 and the tumour microenvironment (TME),5 have been extensively studied at the single-cell level. These studies have resulted in the identification of cellular diversity and its effects within tissues, facilitating the discovery of new therapeutic targets and biomarkers.6, 7 These findings have important implications for drug development, enabling the creation of targeted therapies that enhance effectiveness whilst minimising side effects, ultimately advancing the field of personalised medicine.
In contrast to the widespread use of scRNA-seq in pharmaceutical research, its application in nutraceutical research remains at an early stage.8 Traditional bulk profiling techniques, such as nutrigenomics, transcriptomics, and metabolomics, are regular platforms for comprehensive analyses and are commonly employed in functional food and nutraceutical research.9, 10 Nutrigenomics focuses on the interactions between dietary nutrients and the human genome, assessing gene expression and metabolic functions resulting from dietary intake.11 Clinical or direct-to-consumer applications of nutrigenomics involve identifying genetic predispositions to diseases that can be mitigated or modified through dietary interventions.12 Challenges in understanding the mechanisms involved with this traditional approach include the diverse array of molecules in foods and multi-targeted effects of nutraceuticals. scRNA-seq can overcome these challenges as it reveals individual cell responses and their interactions with various bioactive compounds found in nutraceuticals and functional foods.8, 13, 14 By integrating the cellular pathways influenced by these mixtures, researchers can uncover mechanisms of action and optimise nutraceutical products for enhanced health benefits. This approach lays the groundwork for developing functional foods and personalised nutrition strategies aimed at disease prevention and health promotion. We agree that there is no perfect approach, and all methods have their limitations. The scNRA-seq will add new and non-biased results to help explaining findings and observations from conventional approaches.
An example of this new approach arose from studies of edible mushrooms, including white button mushrooms (WBM), shiitake, and maitake. These fungi have been widely studied for their potential anti-cancer and health-promoting properties.15 Rich in bioactive compounds such as polysaccharides (beta-glucans, glycoproteins) and secondary metabolites (triterpenoids, polyphenolic compounds, amino acids, and lipids), these fungi enhance immune responses and exhibit direct anti-cancer effects.16 Numerous reviews highlight the medicinal potential of these compounds at the molecular level, suggesting that edible mushrooms can play a valuable role in a balanced diet, offering protective benefits against cancer and other chronic diseases.17 They exemplify the concept of “food as medicine,” emphasising the significant impact of dietary choices on health outcomes. However, the often-promoted list of anti-cancer edible mushrooms can be misleading and often lacks solid clinical evidence, that is, a correlation rather than causation. Some of the current evidence comes from epidemiological studies, in vitro experiments, and animal studies. More clinical trials are needed to establish their role in human health, particularly to understand the mechanisms of edible mushrooms as whole foods and assess the long-term effects of regular consumption on disease risk, especially cancer. Unlike synthetic drugs, mushrooms offer a synergistic blend of bioactive compounds, with biological effects arising from a multi-targeted network of food chemicals, which may vary across human cells and tissues. Traditional bulk-analysis techniques, such as bulk RNA sequencing, inflammatory cytokine arrays, and targeted flow cytometry, still dominate research designs, leaving the precise mechanisms through which mushrooms affect health at the molecular and cellular levels largely uncertain.
This review shares our experiences and recent advancements in single immune cell profiling techniques to explore the immunomodulatory mechanisms of WBM in cancer patients. We emphasise key considerations for conducting clinical nutraceutical research with advanced technologies and encourage researchers in the functional foods and nutraceuticals sector to utilise scRNA-seq to bridge knowledge and translational gaps. The novelty of applying scRNA-seq in this context lies in its capacity to reveal the molecular mechanisms underlying the health benefits of these compounds. Whilst scRNA-seq is well-established in pharmaceutical research, this review contextualises it within the unique challenges of nutraceutical research. By highlighting the insights provided by scRNA-seq, we aim to inform researchers about its potential to enhance understanding beyond traditional methods, such as flow cytometry and bulk arrays.
2 “Prior-human evidence” of clinical nutraceutical research: major challenges
According to the US FDA, ‘Prior-Human Evidence’ pertains to nutraceutical/botanical products that have been previously marketed or evaluated in clinical studies, as documented in the literature.18, 19 Unlike dietary supplements such as vitamins or medicinal herbs, nutraceuticals are food-based materials designed for specific medical purposes, making clinical evidence from well-conducted trials essential to ensure their effectiveness and safety.20, 21 Many edible mushrooms, whether consumed as whole foods or in supplement forms such as powders, tablets, or capsules, possess prior human evidence supporting their health benefits. To comprehend the discrepancy between human consumption and clinical effects, we conducted a systematic analysis of clinical trials registered at ClinicalTrials.gov. Common edible mushroom species and/or their derived dietary supplement available on the global market are examined. These include, but are not limited to, farm-grown varieties such as WBMs/brown mushrooms/portabella (Agaricus bisporus), shiitake mushrooms (Lentinula edodes), maitake mushrooms (Grifola frondose), oyster mushrooms (Pleurotus osreatus), king trumpet mushrooms (Pleurotus eryngii), lion's mane mushrooms (Hericium erinaceus), and enokitake mushroom (Flammulina filiformis) as well as some wild-edible varieties like cauliflower mushrooms (Sparassis crispa), Brazilian mushroom (Agaricus blazei Murill), amongst others. These trials involve the use of edible mushrooms for the prevention, treatment, and supportive care of various diseases, and are summarised and reported across categories including cancer, immunity and inflammation, obesity and body weight control, lipemia, glycaemia and cardiovascular health, microbiome and gut health, neurocognition an neuroinflammation. (Figure 1: ‘Prior-Human Evidencde' of Edible Mushrooms as Nutraceuticals for the Prevention, Treatment, and Supportive Care of Various Diseases and Health Conditions’).
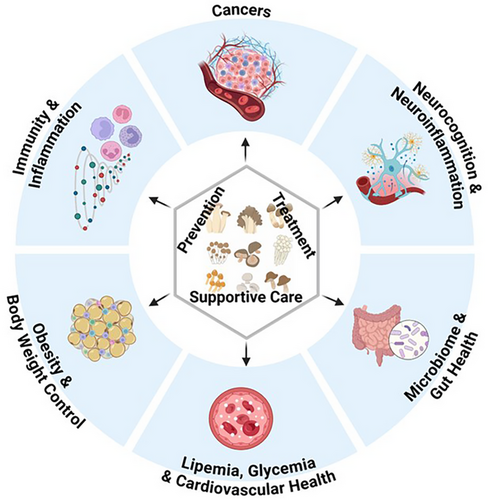
We were particularly interested in the methods and biomarkers used to measure the clinical and biological outcomes. This analysis aimed to summarise the ‘Prior-Human Evidence’ for edible mushrooms in managing various diseases, as observed in current trials and to provide direction for future research. We found 34 relevant clinical trials registered at ClinicalTrials.gov. Amongst these, 13 trials focused on the prevention, treatment, and supportive care of cancers named prostate, breast, ovarian, lung, colorectal, and haematologic cancers (Table 1). Eight trials were designed to evaluate the effects of mushrooms on immunity and inflammation such as vaccination response, immune enhancement, and inflammation management (Table 2). The remaining 13 trials concentrated on other health conditions; amongst them, eight were designed to address obesity and body weight control, lipemia, glycaemia and cardiovascular health, whilst the rest five focused on neurocognition and neuroinflammation in terms of mental health and cognitive improvement (Table 3). Through our review of these studies, we found that common biomarkers include changes in circulating immune cells and inflammatory responses. More specifically, this may involve evaluations of the numbers of the leukocytes and their subgroups (such as neutrophils, myeloid-derived suppressor cells/MDSCs, T and NK lymphocytes) characterised by flow cytometry, as well as the levels of inflammatory cytokines such as high-sensitivity C-reactive protein (hsCRP), IL-4, IL-6, IL-10, IFN-γ, and TNF-α profiled by cytokine array.22-25 Changes in faecal microbiome composition and blood metabolic markers are becoming important biomarkers in nutraceutical studies. Clinical samples collected for outcome measurement may include whole blood, serum or plasma, peripheral blood mononuclear cells (PBMCs), stool, and normal and diseased tissue samples. In terms of methodology and techniques for measuring biomarkers, conventional targeted approaches that measure specific cell types or circulating biomarkers are straightforward to implement clinically because sample collection, processing, and measurement are standardised and well-established in clinical laboratories. These include methods in clinical haematology—including differential white blood cell counts and flow cytometry assays for specific cells—along with approaches in clinical immunology and chemistry, such as cytokine arrays and metabolic panels, which have been predominantly utilised.
| Mushroom | Cancer type | Phases | Study design | Status | Outcome measuring | Results and conclusion | Identifier |
|---|---|---|---|---|---|---|---|
|
White button mushroom (Agaricus bosporus) |
Prostate cancer (biochemically recurrence) | Ib |
Treatment Single Arm, Open Label 36 Participants |
Complete |
|
|
NCT00779168 |
| Prostate Cancer (Biochemically Recurrence & Therapy naive favourable risk) | II |
Treatment Two Arms, Randomised, Open Label 132 Participants |
Recruiting |
|
No results posted | NCT04519879 | |
| Breast Cancer (Postmenopausal Survivors) | I |
Treatment Single Arm, Open Label 16 Participants |
Complete |
|
|
NCT00709020 | |
|
Breast Cancer (Obese Postmenopausal Women at High Risk) |
I |
Prevention Single Arm, Open Label 26 Participants |
Recruiting |
|
No results posted | NCT04913064 | |
| Shiitake mushrooms (Lentinula Edodes Mycelia AHCC) |
Ovarian cancer (patients on adjuvant chemotherapy) |
II |
Prevention randomised, double-blinded placebo-controlled 22 Participants |
Recruiting |
|
Not conclusive larger trial would be needed |
NCT05763199 |
| HPV-positive patients with head and neck squamous cell carcinoma | n/a |
Treatment single arm open label 34 participants |
Not Yet Recruiting |
|
No results posted | NCT06693323 | |
|
Maitake mushrooms (Grifola frondose, Lion's Mane (Hericium erinaceus) AndoSan™ |
Lung neoplasms and breast carcinoma | I |
Supportive care multiple arms, randomised, open label 120 participants |
Complete |
|
No results posted | NCT02603016 |
| Patients with advanced cancer | I |
Treatment single arm, open label 1 participant |
Terminated |
|
No results posted | NCT01200004 | |
|
Colorectal cancer (cancer-related fatigue with AndoSan) |
n/a |
Supportive care single arm, open label 30 participants |
Recruiting |
|
No results posted | NCT06599710 | |
|
Cancer patients (under systemic chemotherapy and/or CD4/6 inhibitors) |
2 |
Treatment single arm, open label 40 participants |
Not yet recruiting |
|
No results posted | NCT06323473 | |
| Myelodysplastic syndrome | II |
Treatment single-arm, open label 45 participants |
Complete |
|
|
NCT01099917 | |
|
Oyster mushrooms (Pleurotus osreatus) MICODIGEST 2.0 |
Colorectal cancer (complications after surgery) | n/a |
Supportive care two arms, randomised, placebo-controlled 144 participants |
Unknown |
|
No results posted | NCT04821258 |
|
Brazilian Mushroom (Agaricus blazei Murill) AndoSan™ |
Multiple myeloma | 2 |
Treatment two arms, randomised, placebo-controlled 39 participants |
Completed |
|
|
NCT00970021 |
| Mushroom | Conditions | Phases | Study design | Status | Outcome measuring | Results and conclusion | Identifier |
|---|---|---|---|---|---|---|---|
|
White button mushroom (Agaricus bosporus) |
Influenza vaccination response (adult, older adult) |
n/a |
Prevention multiple arms, randomised placebo-controlled 105 participants |
Recruiting |
|
No results posted | NCT06041867 |
| Shiitake mushrooms (Lentinula Edodes) |
Promotion of immunity (health adult) |
n/a |
Prevention two arms, randomised placebo-controlled 52 participants |
Completed |
|
|
NCT01398176 |
| Shiitake mushrooms (Lentinula Edodes Mycelia AHCC) | Clearance of high risk-HPV infections | n/a |
Treatment randomised 60 participants |
Recruiting |
|
No results posted | NCT04633330 |
| Eradication of HPV infections | II |
Treatment randomised double-blind, placebo control 50 participants |
Completed |
|
|
NCT02405533 | |
|
Shiitake mushrooms (Lentinula Edodes) Lion's Mane (Hericium erinaceus) Maitake mushrooms (Grifola frondose) Myco-Digest |
Inflammatory bowel diseases | n/a |
Supportive care two arms, randomised, open label 100 participants |
Unknown status |
|
No results posted | NCT04329481 |
|
Oyster mushrooms (Pleurotus osreatus) |
Neuroinflammation, cognitive change (older adult) |
n/a |
Treatment multiple arms, randomised cross-over 33 participants |
Completed |
|
|
NCT05594329 |
|
Cordyceps mushrooms (Cordyceps militaris) |
Neuroinflammation, emotional problem (adult, older adult) |
n/a |
Treatment two arms, randomised double-blind placebo-controlled 80 participants |
Completed |
|
No results posted | NCT04002219 |
| Cauliflower mushroom |
Promotion of immunity (health adult) |
n/a |
Prevention two arms, randomised double-blind placebo-controlled 105 participants |
Recruiting |
|
No results posted | NCT06041867 |
| Mushroom | Conditions | Phases | Study design | Status | Outcome measuring | Results and conclusion | Identifier |
|---|---|---|---|---|---|---|---|
|
White button mushroom (Agaricus Bosporus) Shiitake mushrooms (Lentinula Edodes) |
Postprandial lipemia and glycemia (adult following a high-fat meal) |
II |
Treatment randomised, double-blinded placebo-controlled 57 participants |
Completed |
|
|
NCT05619952 |
|
White button mushroom (Agaricus Bosporus) Oyster mushrooms (Pleurotus osreatus) |
Healthy eating (adult, older adult) |
n/a |
Screening randomised crossover assignment 7 participants |
Completed |
|
No results posted | NCT04257201 |
| Shiitake mushrooms (Lentinula Edodes) | Moderate hyperlipidemia | II |
Treatment randomised, double-blinded placebo-controlled 57 participants |
Completed |
|
|
NCT03550287 |
| Cholesterolemia and oxidative stress levels |
Treatment randomised, double blind and placebo controlled 68 participants |
Completed |
|
|
NCT04186780 | ||
|
Oyster mushrooms (Pleurotus osreatus) |
Antiretroviral treatment (ART) induced hyperlipidemic (adult, older adult) |
n/a |
Treatment non-randomised single arm open label 20 participants |
Completed |
|
|
NCT00069524 |
| Metabolic syndrome | n/a |
Prevention randomised, open label cross-over 19 participants |
Completed |
|
No results posted | NCT04444219 | |
|
Healthy eating (adult, older adult) |
n/a |
Screening randomised parallel assignment 53 participants |
Completed |
|
|
NCT04259229 | |
|
Lion's mane (Hericium erinaceus) |
Parkinson disease (adult, older adult) |
n/a |
Supportive care randomised, placebo-controlled 80 participants |
Unknown status |
|
No results posted | NCT04428983 |
|
Cognitive decline, mild (adult, older adult) |
n/a |
Prevention randomised, double-blind placebo-controlled 40 participants |
Completed |
|
No results posted | NCT04939961 | |
| Premenstrual syndrome | n/a |
Treatment randomised, placebo-controlled 105 participants |
Unknown status |
|
No results posted | NCT05443477 | |
| Tinnitus/ hearing impairment | n/a |
Supportive care randomised placebo-controlled 80 participants |
Unknown status |
|
No results posted | NCT03632512 | |
|
Mild or medium dementia cognitive decline |
n/a |
Supportive care randomised, double-blind placebo-controlled 68 participants |
Completed |
|
higher CASI, MMSE, and IADL scores and achieved a better contrast sensitivity in patients with mild AD. |
NCT04065061 | |
| Edible mushroom meal |
Obesity and body weight control (adult, older adult) |
Treatment two arms randomised, open label 100 participants |
Completed |
|
No results posted | NCT01177085 |
- Note: AHCC is a mixture of components derived from cultured mycelia of Lentinula edodes, also known as “Shiitake”. AndoSan is a specialised liquid food product that includes fermented extracts from the fungi Agaricus subrufesence (Agaricus blazei Murill, 82%), Hericium erinaceus (15%), and Grifola frondose (3%) as the main ingredients. MICODIGEST 2.0 is a dietary supplement composed of fungal extracts of Agaricus blazei, Grifola frondosa, Hericium erinaceus, Cordyceps sinensis, Inonotus obliquus, Pleurotus ostreatus, Polyporus umbellatus and Lentinula edodes, with potential anti-inflammatory and immunomodulatory activities. Myco-Digest is a dietary supplement composed of fungal extracts of Lentinula edodes, Hericium erinaceus, Coriolus versicolor, Inonotus obliquus with potential anti-inflammatory and immunomodulatory activities.
The design of clinical trials varies based on the biomarkers and surrogate endpoints selected by investigators. Trials may focus on disease modification, recovery, and other endpoints defined by different biomarkers.26 Outcome measures for nutraceutical interventions often include patient-reported data and biomarkers that must be clinically relevant and assessed using reliable methods. However, accurately identifying a biomarker that reflects the mechanisms of action or biological and clinical responses of nutraceuticals can be extremely challenging due to the complex compositions and chemical properties of these agents. As a result, a specific biomarker used for trial evaluation may only capture the biological activity of the one component within the nutraceutical.21, 27 For example, multi-spectral flow cytometry is known for its single-cell resolution and capacity to measure cell population changes. However, its capabilities are often limited to the detection of predefined proteins.28 For drugs with defined mechanisms, chemical profiles, and targeted molecular/cell types, the multi-spectral flow cytometry is effective for assessing treatment responses. However, for nutraceutical products that contain a diverse array of molecules and have undefined mechanisms, utilising simple targeted approaches may lead to overlooking varying reactions across diverse cell types within a tissue, resulting in biased conclusions.29, 30 Studies involving foods show that traditional methods such as flow cytometry or cytokine arrays are inadequate for detecting simultaneous changes amongst the different types of cells involved in the response.
Flow cytometry has several limitations that may not adequately address the needs of nutraceutical research. First, it does not provide comprehensive gene expression profiles at the transcriptomic level. This means that it may miss critical information regarding the expression of genes not associated with the measured markers, limiting the understanding of cellular responses to nutraceuticals. Additionally, whilst it is excellent for assessing cell population changes, flow cytometry lacks the ability to evaluate the functional readouts at the level of mRNA, which is crucial for understanding how diverse bioactive compounds interact within individual cells. This limits its capacity to offer insights into the mechanisms through which nutraceuticals exert their effects. Lastly, in contrast to single-cell techniques, flow cytometry averages the signals across populations, potentially masking important variations amongst individual cells. Therefore, advanced techniques like scRNA-seq are essential for accurately analysing complex cell mixtures at the single-cell and single-molecule levels, ultimately enabling the identification of biomarkers that determine nutraceutical efficacy and response.
3 Application of single-cell RNA sequencing to clinical nutraceutical research: technological advances
Given that nutraceuticals usually consist of mixtures of various ingredients and therefore the potential for multiple targets, selecting appropriate endpoints and biomarkers for investigations can be difficult. Additionally, having a reliable biomarker linked to the biological endpoint of interest is crucial for the success of a clinical trial. Consequently, these trials frequently require advanced analytical techniques to provide insights into the desired outcomes.21, 29, 31 As an example, in our Phase I clinical trial (NCT00779168) involving prostate cancer (PCa) patients consuming WBM derived tablets, WBM consumption resulted in at least two significant bioactivities. First, 13 out of 36 participants experienced a decrease in prostate-specific antigen (PSA) levels without changes in serum testosterone, indicating anti-androgenic activity against hormone-responsive tissues. Second, patients with PSA declines showed a reduction in circulating MDSCs (CD33+HLA-DR−) as measured by multispectral flow cytometry, suggesting an immune regulatory effect of WBM.23 Additionally, several preclinical studies have demonstrated WBM's immunoregulatory effects on dendritic cells, natural killer cells, and other innate immune cells using individual cell cultures or animal models.32, 33 Collectively, all the “Prior-Human Evidence” suggests that WBM demonstrates multi-target effects against various cells and pathways.
To fully describe the effects of WBM on various cell types, we are utilising single immune cell profiling analysis of whole blood samples from patients in our ongoing Phase 2 clinical trial (NCT04519879) in PCa. Single-cell techniques enabled us to confirm that MDSCs were one of the major cell types responding to the WBM treatment, along with changes in other cell types, such as cytotoxic T cells and NK cells.34 Through single-cell transcriptomics analysis, we explored the molecular pathways affected by the WBM treatment and found that STAT3 and IRF1, key transcriptional regulators of MDSC expansion and survival, were suppressed, potentially impairing their immunosuppressive functions. Upon further investigation of immune checkpoint expression on T cells and NK cells, we observed elevated levels of PD-1 following WBM treatment. This observation led us to propose and conduct a proof-of-concept study combining WBM with PD-1 therapy to enhance responsiveness to immune checkpoint inhibitors.34 Our phase I and phase II studies have shown that as compared to conventional flow cytometry or bulk analysis, scRNA-seq excels in capturing the heterogeneity within cell populations, enabling an exploration of how different cell types respond to nutraceuticals at a molecular level. scRNA-seq can also provide a detailed picture of the gene expression at the single-cell level, allowing researchers to assess how individual cells respond to a complex mixture of bioactive compounds. This is crucial for understanding the nuanced effects that a single molecule or a mixture may have on various cell types. Most importantly, scRNA-seq enables the identification of novel transcripts and regulatory mechanisms that may play a role in the response to complex nutraceuticals. These insights can lead to the discovery of potential biomarkers for efficacy and safety tailored specifically to nutraceutical applications and clinical trial design.
Our understanding of the immune system's complexity and heterogeneity, which includes various cell types across primary and secondary lymphoid organs, has been significantly advanced through single-cell studies.35 These studies have identified specific immune cell subsets and their developmental paths, emphasising the importance of studying immune-boosting foods such as edible mushrooms at a cellular level to fully harness their therapeutic potential. In cancer research, inter- and intra-tumoral cellular heterogeneity and transcriptional diversity pose significant challenges.2 Single-cell sequencing techniques have enhanced our knowledge of key areas such as premalignant invasion, the TME, metastasis, and therapeutic resistance.36 scRNA-seq has uncovered diverse drug effects; for instance, etoposide influences proliferating tumour cells, whereas panobinostat impacts not only tumour cells but also the immune microenvironment in surprising ways.37 In addition to the above-mentioned applications, the human microbiome which comprises a vast community of microorganisms, including bacteria, viruses, fungi, and other microbes, is another system that can significantly influence the efficacy of nutraceuticals.38 For instance, certain bioactive compounds in nutraceuticals are metabolised by gut microbiota, leading to bioactive metabolites that may contribute to the health benefits associated with those compounds. A relevant example is the impact of polyphenols found in foods like blueberries or green tea. These compounds can be metabolised by specific gut bacteria into phenolic acids, which have been shown to exert antioxidant, anti-inflammatory, and even anti-cancer effects.39 Whilst traditional approaches to studying the microbiome often rely on bulk sequencing or metagenomics,40 these methodologies have limitations in resolving the complexities of microbial communities at the individual cell level. Single-cell approaches have significantly enhanced the understanding of the human microbiome by allowing researchers to examine the genetic and functional characteristics of individual microbial cells within complex communities. scRNA-seq provides insights into microbial composition, function, and dynamics across various environments, such as the human gut, skin, and oral cavity. Crucially, scRNA-seq can identify therapeutic microbes that respond positively to specific nutraceuticals, informing personalised nutrition strategies. By employing these methodologies, researchers can determine which microbes are beneficial and how they contribute to health outcomes, paving the way for targeted interventions.41 In our Phase 2 clinical trial with WBM, we also designed a study to compare the stool microbiome characteristics of PCa patients treated with or without WBM against those of control patients.
Single-cell sequencing is a powerful technique. However, obtaining suitable clinical samples can also be problematic. Whilst blood and stool are generally accessible, acquiring tissue often requires invasive procedures such as surgeries or biopsies. Accessing other tissues, such as those from the brain, liver, or intestines, poses significant logistical and ethical challenges.42 Additionally, single-cell analysis usually involves dissociating tissues into individual cells, which results in a loss of spatial context crucial for understanding cell organisation and interactions, especially in tumours. This dissociation can alter the natural state of cells, potentially affecting gene expression profiles and leading to data artefacts, with fragile cell types likely being underrepresented.43
In contrast, spatial omics complement single-cell sequencing by preserving the spatial arrangement of cells within tissues. This approach allows direct analysis of tissue sections, maintaining the natural contextual state of cells and providing more accurate gene expression data. Spatial omics can simultaneously assess multiple molecular layers (e.g., RNA and proteins), offering an integrated view of the tissue environment that enhances understanding of complex biological processes. This spatial data facilitate intuitive interpretations of the gene expression related to specific tissue structures, improving insights into physiological and pathological states.44, 45 For example, an active research area is the application of spatial omics and single-cell RNA sequencing (scRNAseq) in functional foods and nutraceuticals within neuroscience. scRNAseq provides an understanding of the heterogeneity amongst brain cells and reveals functional differences.46, 47 Recent studies indicate that functional foods rich in flavonoids, such as blueberries and dark chocolate, may provide neuroprotective effects and aid in slowing neurodegenerative diseases.48 Spatial analysis, particularly through spatial transcriptomics has been crucial in these investigations, enabling researchers to analyse brain tissue from mouse models of Alzheimer's and Parkinson's diseases. This technique has resulted in the creation of high-resolution maps that reveal how flavonoid consumption affects the gene expression in key areas, such as the hippocampus.49 Spatial analysis would help to uncover interactions between cell types—neurons, astrocytes, and microglia—in response to the flavonoid treatment, helping to assess how these compounds influence inflammatory responses and neuronal survival. Moreover, by integrating spatial transcriptomic data with bioinformatics, researchers have identified specific pathways activated by flavonoids, including those related to antioxidant defenses and neurotrophic factors. Ultimately, these findings can be translated to human studies by analysing postmortem brain samples to correlate gene expression patterns with dietary history, potentially linking functional food intake to cognitive function and disease progression.50, 51
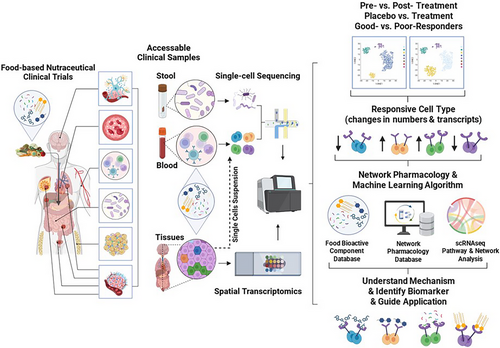
In summary, single-cell analysis, along with spatial omics, is poised to play a crucial role in the future of nutraceutical research, presenting significant opportunities to improve human health through more effective and personalised nutritional interventions. Whilst both techniques offer groundbreaking insights into physiology and disease biology at the single-cell level, each presents unique obstacles that researchers must navigate. Existing review papers have thoroughly discussed the challenges and limitations of scRNA-seq and spatial omics in basic and translational research.52 Common concerns include technical issues such as dropout events, sampling bias, technical complexity, resolution limitations and high costs.53 In clinical nutraceutical research, these same challenges persist. Nevertheless, progress in technology and data analytics is expected to address these issues, further expanding the use of single-cell omics in these fields. To establish a clearer relationship between scRNA-seq data and the effects of nutraceuticals, we propose the following approaches to be considered: first, integration of multi-omics data: we propose to combine scRNA-seq data with other omics approaches, such as proteomics and metabolomics, to create a comprehensive overview of how various bioactive compounds interact within cells. This multi-layered biological information will enhance our understanding of the complex interactions influencing cellular responses to nutraceuticals.54 Second, pathway and network analysis: we suggested utilising network-based machine-learning strategy tools on scRNA-seq data to identify key signalling pathways activated by different molecules found in nutraceuticals. By mapping gene expression changes to established biological pathways, we aim to clarify how diverse compounds contribute to the overall effects of nutraceuticals. Like traditional Chinese herbal medicine, which also encompasses a wide array of chemical profiles, network pharmacology has profoundly transformed research within this discipline.55 In a related context, researchers in the field of functional foods have pioneered a network-based machine learning algorithm designed to predict cancer-fighting molecules in foods, thereby facilitating the development of next-generation cancer-preventive and therapeutic nutritional strategies.56, 57 Third, comparative studies: we recommend that future research incorporate comparative studies that juxtapose cell responses to nutraceuticals with those to pharmaceuticals and other conditions. Such comparisons will help elucidate the unique contributions of nutraceuticals and identify specific molecular mechanisms distinct from those triggered by single pharmaceutical agents. Fourth, hypothesis generation: we emphasise that scRNA-seq can be a powerful tool for the hypothesis generation. By uncovering novel gene expression patterns in response to a mixture of bioactive compounds, researchers can develop new research questions and targeted experimental designs to further investigate the underlying mechanisms. The ongoing integration of these technologies promises to drive breakthroughs in medical and health sciences. An overall view of our proposal to integrate single-cell and spatial techniques into clinical nutraceutical studies to advance our understanding of these important applications is shown in Figure 2: ‘Integrating Single-cell Sequencing and Spatial Transcriptomics into Clinical Nutraceutical Research Enhances Understanding of Mechanisms, Identifies Biomarkers, and Guides Applications.’
4 CONCLUSION AND PERSPECTIVE
The widespread consumption of nutraceuticals by the public and many patients, often without a clear understanding of their functions, mechanisms, activities, or potential toxicity, highlights the need for comprehensive clinical evidence-based studies. These studies are essential to inform consumers and healthcare providers of the benefits and risks associated with nutraceutical use. By designing and conducting such research, we can ensure that nutraceuticals are used safely and effectively, maximising health benefits whilst minimising potential harm. It is crucial to bridge the gap between popular limited knowledge and scientific understanding through rigorous investigations.
- Phase 1: Preliminary research and feasibility studies. Initially, we will identify and validate specific nutraceutical compounds that have established biological impacts through initial omics analyses. Pilot studies will then be conducted using scRNA-seq and spatial omics on various nutraceutical samples, such as blood, stool, or tissues, to assess feasibility.
- Phase 2: Biomarker discovery. In this phase, data from scRNA-seq and spatial omics will be utilised to discover potential biomarkers linked to health outcomes influenced by nutraceuticals. Collaborations with clinical research teams will validate these biomarkers in larger, diverse cohorts to ensure their robustness and reproducibility.
- Phase 3: Clinical validation. Large-scale clinical trials will be designed and executed to evaluate the efficacy of identified nutraceuticals, using the discovered biomarkers as endpoints. The trials will assess the impact on health outcomes, with follow-up studies to monitor long-term effects.
- Phase 4: Regulatory acceptance. Comprehensive data will be compiled to demonstrate the safety and efficacy of nutraceuticals, guided by biomarkers identified through scRNA-seq and spatial omics. Collaboration with regulatory agencies will help establish guidelines for biomarker-driven evaluations of nutraceuticals.
- Phase 5: Implementation and standardisation. In the final phase, standardised protocols will be developed to integrate scRNA-seq and spatial omics technologies into routine nutraceutical research. Additionally, education and training programs will be promoted for researchers and clinicians to effectively utilise these technologies in practice.
By following this roadmap, we aim to bridge the gap between advanced omics technologies and clinical nutraceutical research, facilitating the discovery of biomarkers and enabling regulatory pathways that will ultimately enhance public health. Looking ahead, it is crucial for researchers and industry professionals to adopt single-cell technologies and incorporate them into clinical nutraceutical studies. This integration could enhance our understanding and the translational potential of nutraceuticals for managing diseases and promoting health.
AUTHOR CONTRIBUTIONS
Conceptualisation: Xiaoqiang Wang and Shiuan Chen. Writing-original draft preparation: X.Q. Writing-review and editing: Yin S. Chan, David Sadava, and Shiuan Chen. Visualisation: Xiaoqiang Wang. Supervision: Shiuan Chen. Project administration: Shiuan Chen. Funding acquisition: Shiuan Chen. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by NIH CA227230 (Chen). Analytic core service was supported by NIH P30CA033572.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.




