Thyroglobulin mRNA ectopic expression was an excellent marker to detect lymph node metastasis for patients with papillary thyroid cancer
Abstract
Objective
To investigate whether the mRNA expression of thyroglobulin (TG) and TSH receptor (TSHR) in lymph node could be used to diagnose lymph node metastasis in patients with papillary thyroid cancer (PTC).
Subjects and methods
Around 156 paraffin samples of lymph nodes from 89 patients with PTC after surgery were collected, and the expressions of TG and TSHR mRNA were detected by nested qPCR.
Results
Compared with the results of confirmed histopathology, 86 out of 87 tissues of lymph nodes metastasis ectopically expressed TG mRNA, while 66 out of 69 tissues of non-metastasis lymph nodes did not express the TG mRNA. The specificity and sensitivity of TG mRNA measurement for detecting the lymph node metastasis were 95.65% and 98.85%, as effective as the first postoperative histopathology. However, the specificity and sensitivity of TSHR mRNA measurement were 92.75% and 85.06%, respectively. The accuracy of TG, TSHR mRNA measurement and the first postoperative histopathology were 97.44%, 88.46% and 95.51%, with the positive predictive rate (PPR) 96.63%, 93.67%, and 98.78%, respectively, negative predictive rate (NPR) 98.51%, 83.12% and 91.89%, respectively, as well as Youden's index 0.95, 0.78 and 0.92.
Conclusions
TG mRNA ectopic expression in the lymph node might be an excellent marker to diagnose the lymph node metastasis for patients with PTCs.
1 INTRODUCTION
Papillary thyroid cancer (PTC), derived from follicular–epithelial tissues, as a subclass of well-differentiated thyroid cancer (DTC), constituted approximately 80% of patients with thyroid cancers, age-standardised incidence of which were up to 6.6/105 in 2020 in the world.1, 2 Most patients have an excellent prognosis with the 10-year survival rates above 90%,3 and recurrence rate up to 5%–40%, most involving regional lymph node.4
Biomarker was a main research aspect in predicting diagnosis of PTC, such as RET/PTC rearrangement, RAS and BRAF mutations,5 ABR, AHNAK2, GPX1, TPO,6 miR-146b,7 miR-221,8 miR-222-3p,9 heat shock protein 70 (HSP70), peroxiredoxin (PRDX) and an isoform of S100 protein (S100A6 protein),10 alpha1-antitrypsin (A1AT),11 telomerase,12 which can partly explain the mechanism of development, invasion, and/or metastasis of the disease. Targeted therapy, including BRAF inhibitors,13 Lenvatinib,14 tyrosine kinase inhibitors,15 Pan-Class I PI3K Inhibitors,16 was increasingly used to manage refractory PTC, and more research should be needed to guide the precision therapy in the future.
Fine needle aspiration and cytology (FNAC) was very important to diagnose PTC,17 however, according to the latest diagnostic criteria—The 2017 Bethesda System for Reporting Thyroid Cytopathology (BSRTC),18 15% and 20% samples have not been make a definite diagnosis.19 Many risk factors20-23 have been found, such as age, sex, the size and number of nodules, but most were inconsistent except history of radiation.24, 25 Possible treatments, including surgical resection—the most effective treatment, postoperative radioiodine (RAI) as adjuvant therapy, and thyrotropin (TSH) suppression, depend on the individual involved.
Histopathology after surgery was used as gold standard for diagnosis of thyroid cancer and lymph node metastases. Involvement of lymph nodes was pathologic features for pathologists to determine Tumor Node Metastasis (TNM) stages according to the recommends by the American Joint Committee on Cancer (AJCC). The lymph nodes metastasis, combined with the size of tumour, gross extrathyroidal invasion, distant metastasis and genetic mutation might be the best predictor of mortality.26 To ensure an adequate evaluation of lymph node status, 6, 8 and 18 nodes may be enough to provide adequacy for lymph node metastasis in patients with T1b, T2 and T3 disease, respectively.27
Thyroglobulin (TG) is specifically expressed in thyroid follicular cells and could release to the circulation. The concentration of the TG protein, detected by RAI, has been used as an indicator to monitor the residue, recurrence, and metastasis of the patient with PTC after total thyroidectomy,28 as well as to improve the diagnostic accuracy of metastasis in lymph node fine-needle aspiration (FNA) washout.29 A series of factors affecting the detected results of TG protein, such as different detection methods (especially immunoassays and radioimmunoassays),30 different reagent kits,31 and interference from heterophilic antibodies,32 were taken into account when evaluating the lymph node metastasis, as well as anti-TG autoantibodies (TgAb) commonly causing falsely low serum TG measurements.33 Serum TG cannot distinguish between benign and malignant thyroid nodules because the level can be elevated in various thyroid diseases, such as goiter, inflammation or injury of thyroid tissue, and hyperthyroidism.34 Given that TG protein could release from thyrocytes to the circulation, it showed lower specificity if TG protein levels in neck lymph node fine needle aspiration washout fluid (FNA-Tg) have been used as marker to detect cervical lymph node metastasis in patients with PTC. In fact, the level of FNA-Tg ≥20 ng/mL used as a cutoff values to detect the lymph node metastasis only exhibited 66.7% specificity.35 Thus, it is tempting to presume that measurement of the mRNA level in tissues of the neck lymph node might be a best marker to detect the cervical lymph node metastasis in patients with PTC due to the Tg mRNA specifically expressed in the invasion thyrocyte.
In this study, the mRNA level of TG and TSHR were measured in 156 formalin fixed paraffin embedded (FFPE) samples of lymphonodes from 89 patient with PTC by nest PCR. Compared with the confirmed histopathological results, the specificity and sensitivity of TG exhibited 95.65% and 98.85%, as effect as the first postoperative histopathology. The specificity and sensitivity of TSHR were 92.75% and 85.06%, respectively. These data suggested that ectopic expression of TG mRNAs may serve as best maker to indicate the cervical lymph node metastasis in patient with PTCs.
2 MATERIALS AND METHODS
2.1 Patients
The 156 FFPE tissues of lymph nodes had been recruited from 89 patients with PTC who underwent thyroidectomy and neck dissection at Linyi People's Hospital from April 2022 to May 2023. The tissues of lymph nodes were fixed in 4% polyformaldehyde within 2 h after surgical removal. The samples were sliced into 2 mm tissue blocks and embedded in paraffin for sections. The slides have been examined by two pathological experts under microscopy and then given postoperative histopathologic diagnosis. Five slides for each FFPE samples of metastasis lymph nodes or non-metastasis lymph nodes were collected for detecting the TG and TSHR mRNA expression. The study was conducted in accordance with the ethical principles of WMA Declaration of Helsinki and approved by the ethics boards of Linyi People's Hospital (202401-H-025).
2.2 Extraction of RNA from formalin fixed paraffin embedded samples
The FFPE samples of cervical lymph nodes were deparaffinised with xylene and rehydrated through ethanol (100%). The samples were digested with proteinase K, incubated at 55°C for 1 h with mild agitation and subsequently at 80°C for an additional 15 min. Extraction of RNA from the FFPE samples was performed using RNAprep Pure FFPE Kit (DP439, Tiangen Biotech Co., Ltd, Beijing) according to the manufacturer's instruction. The extracted RNA elute was stored at −80°C. RNA quantification was performed by NanoDrop (Thermo Scientific, Inc., Waltham, MA, USA).
2.3 Detection of thyroglobulin or TSH receptor expression by qPCR with nest PCR
200 ng total RNA was used to reverse transcribe cDNA according to the manufacturer's instruction (RR047B, Takara Bio Inc.). Nest PCR was performed using two sets of primers. The first PCR were run with the following conditions: 50°C for 5 min and then 95°C for 5 min; 15, 20, 25 and 30 cycles at 95°C for 30 s, 60°C for 30 s and 72°C for 20 s, respectively. Final, keeping at 72°C for 10 min. After the first PCR amplification, the products were diluted 5 times and 1µL was used as the second qPCR template and the reactions were run on the ABI 12K machine (Thermo Scientific, Inc., Waltham, MA, USA) with the following conditions: 50°C for 2 min and then 95°C for 30 s. 50 cycles at 95°C for 10 s, 60°C for 34 s. TG and TSHR probes were 3′MBG- 5′FAM labelled and the housekeeping gene GAPDH probes were 3′MBG-5′VIC labelled. The GAPDH was used as reference to reflect the effectiveness of the reaction systems. The primers and probes sequences were listed below: TG-outer-F/R: TGCTACATGGTATTACTCTCTGG/GCCGTAGTTTTCAGGAGCATG;
TSHR-outer-F/R:GCCCAATATTTCCAGAATCTACG/AACTTTGGTCAGGTCAGGGA;
GAPDH-outer-F/R:ATCAAGAAGGTGGTGAAGCAGG/CATACCAGGAAATGAGCTTGACAA;
TG-inner-F/R: CACGGATGACTATGCCTCCTT/GGTACATGAAGACGTTTCCTCG;
TSHR-inner-F/R: GCTGGAATCACACTCCTTCTAC/AGGGCATCAGGGTCTATGTA;
GAPDH-inner-F/R: CTCAAGGGCATCCTGGGCTA/CCAGCGTCAAAGGTGGAGGA;
GAPDH probe: CACTGAGCACCAGGTGGTCTCCT;
TG probe: AATGCCACCCGGGACTACTTTATCATC;
TSHR probe: AGTGACTCACATAGAAATTCGGAATACCAGG.
2.4 Result determination strategy
Only the cycle threshold (CT) value of GAPDH < 30 was regarded as an effective amplification. The CT value of TG or TSHR < 30 was defined as positive for measurement of TG or TSHR mRNA expression. When the molecular diagnosis was inconsistent with the first postoperative histopathologic diagnosis, the lymph node slices were re-evaluated by two pathologic experts by microscopy according to strict histologic criteria and these results were defined as the confirmed histopathology diagnosis. The specificity and sensitivity of molecular diagnosis were gotten by comparing with the final pathologic diagnosis reports.
2.5 The hematoxylin–eosin and immunohistochemistry stain
Paraffin-embedded lymph node sections were deparaffinised and treated with a hydrochloric acid-based antigen retrieval solution. The hematoxylin–eosin (H&E) stain was done briefly as follows: dip the slide into a Coplin jar containing Mayer's hematoxylin and agitate for 30 s. Rinse the slide in H2O for 1 min. Stain the slide with 1% eosin Y solution for 10–30 s with agitation and then dehydrate the sections with two changes of 95% alcohol and two changes of 100% alcohol for 30 s each. Extract the alcohol with two changes of xylene and add one or two drops of mounting medium and cover with a coverslip. The immunohistochemistry (IHC) stain was done briefly as follows: endogenous peroxidase was suppressed with a solution of 3.0% H2O2 in methanol for 10 min, and then blocked with 2% bovine serum albumin (BSA) for 1 h. Sections were incubated overnight at 4°C in mouse anti-TG monoclonal antibody (Proteintech, Cat No:60272-1-Ig, 1:200). The sections were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Dako) at room temperature for 1 h. Immunoreactivity was visualised with 0.2% 3,3′-diaminobenzidine (DAB).
2.6 Statistical analyses
The t-test (normal distribution data) and Mann−Whitney test, as well as Yates's correction for continuity (non-normal distribution data) were used to compare continuous data. χ2-test was used to compare categorical data. The chosen statistical test for each analysis is indicated in figure legends. A p-value of < .05 was considered significant. The Statistical Package for Social Science version 25.0 (SPSS, Inc., New York, NY, USA) was used for data analyses.
3 RESULTS
3.1 Clinical characteristics of patients
There were 89 PTC patients incorporated in this study, with mean age of 42.27 ± 12.01 years and more females than males (77.53% vs. 22.47%). A total of 156 paraffin tissues of lymph nodes from the 89 PTC patients were obtained, of which 82 samples were metastatic lymph nodes based on the first postoperative histopathology. The clinical and pathological information were provided in Table 1. There was higher FT3 level in men than that in women before the surgery (5.59 vs. 5.08 pmol/L, p = .003), while no difference in the FT4 level between males and females (15.52 vs. 15.16 pmol/L, p = .19). There were 41 patients (8 males) with bilateral thyroid lesions, and 48 (12 males) with unilateral thyroid lesions, showing no significant difference in the sex distribution (p = .54). Out of 48 patients with unilateral lesions, 35 patients were performed the ipsilateral-side neck lymph nodes dissection, without chance to evaluate the contralateral lymph node status. The average number of resected lymph nodes was 18.69 per patients. The paraffin samples of lymph nodes with metastasis or without metastasis were recruited from the same patient with PTC for further research.
| Variable | Male (n = 20) | Female (n = 69) | Total (n = 89) | p-Value | Statistical method |
|---|---|---|---|---|---|
| Age (Mean ± SD)(y) | 43.15 ± 12.28 | 42.01 ± 12.00 | 42.27 ± 12.01 | .72 | t-test |
| FT3[M(P25,75)](pmol/L) | 5.59(5.44,6.10) | 5.08(4.71,5.56) | 5.18(4.73,5.63) | .03 | Mann–Whitney U-test |
| FT4[M(P25,P75)](pmol/L) | 15.52(12.52,17.75) | 15.16(13.61,16.42) | 15.46(13.52,16.94) | .19 | Mann–Whitney U-test |
| Macrocarcinoma[M(P25,P75)](cm) | 1.10(0.68,1.70) | 1.50(0.80,2.00) | 1.30(0.80,2.00) | .28 | Mann–Whitney U-test |
| Bilateral lesions | 8 | 33 | 41 | .54 | Chi-square test |
| Unilateral lesion | 12 | 36 | 48 | ||
| Ipsilateral-side lymph nodes resected | 10 | 25 | 35 | .58 | Yates's correction for continuity |
| Multi-lesion resected | 2 | 11 | 13 |
3.2 Combination of qPCR with nest PCR increases the sensitivity for detection of TG and TSHR mRNA expression in paraffin samples
We established a method to detect mRNA expression of TG and TSHR in FFPE samples of cervical lymph nodes from patient with thyroid cancer by qPCR with nest PCR in this study. Initial experiments were performed using six well-defined metastatic or non-metastatic cervical lymph nodes FFPE samples respectively and 200 ng total RNA was used as templates to do reverse transcription of cDNA. The amplification cycles of the first round PCR were used as 15, 20, 25 and 30 cycles, and then 1 µL of 5 times diluted first round PCR products were used as templates to amplify the TG, TSHR and GAPDH mRNA in lymph nodes by qPCR. We found that when the amplification cycles of the first round PCR were 15, some of the CT values of GAPDH were above or around 30, which were inclusive for an internal reference. When the amplification cycles of the first round PCR are 25 times and cut-off value was set as below 30, the qPCR results of TG and TSHR were 100% consistence to the pathologic diagnosis and CT values of GAPDH were approximately 19–25, which was neither too high nor too low, and suitable to evaluate the transcription levels of other genes (data not shown). So, the method was established as the amplification cycles of the first round PCR were 25 and the cut-off CT value of TG or TSHR expression to detect the cervical lymph node metastasis was set as below 30. Subsequently, we used this approach to detect the expression of TG and TSHR in the FFPE samples of the lymph node from patients with PTC.
3.3 The measurement of thyroglobulin and TSH receptor mRNA ectopic expression to detect the lymph nodes metastasis
Among the 82 FFPE samples of metastatic lymph nodes according to the first postoperative histopathology, 80 metastatic lymph nodes have been detected the ectopic expression of TG mRNA, only two metastatic lymph nodes were negative for the expression of TG mRNA (Table 2). Moreover, one out of the two metastatic lymph nodes without TG mRNA expression has been diagnosed as truly metastasis in the confirmed histopathology by two pathological experts. Notably, 9 out of the 74 FFPE samples without lymph nodes metastasis according to the first postoperative histopathology have been detected the ectopic expression of TG mRNA. Interestingly, out of the 9 FFPE samples without lymph nodes metastasis by the first postoperative histopathology but TG mRNA tested positive, 6 samples have been re-interpreted as truly metastasis in the confirmed histopathology by two pathological experts (Table 2, Figure 1), 3 of them have also been verified by immunohistopathology using antibody to TG protein (Figure 1).
| The first postoperative pathologic diagnosis | Confirmed pathologic diagnosis | TG expression | Total Number | |
|---|---|---|---|---|
| Positive | Negative | |||
| Metastasis | Metastasis | 80 | 1 | 81 |
| Non-metastasis | Metastasis | 6 | 0 | 6 |
| Metastasis | Non-metastasis | 0 | 1 | 1 |
| Non-metastasis | Non-metastasis | 3 | 65 | 68 |
| Total number | 89 | 67 | 156 | |
- Abbreviation: TG, Thyroglobulin.
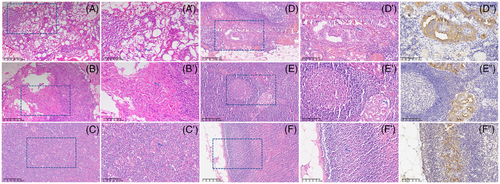
When the confirmed histopathological diagnosis as gold standard, 86 out of the 87 metastatic lymph nodes exhibit TG mRNA expression, with the sensitivity 98.85%. While out of 69 non-metastatic lymph nodes, 66 did not expressed the TG, with the specificity of TG mRNA marker 95.65% (Table 3). There is one sample, diagnosed as metastatic lymph nodes by the first postoperative histopathology but TG mRNA negative, which was re-interpreted as non-metastatic by confirmed histopathology. The accuracy of TG mRNA marker was 97.44%, the positive predictive rate (PPR) and negative predictive rate (NPR) of TG mRNA marker reached up to 96.63% and 98.51%, respectively, as well as Youden's index 0.95 (Table 3). Notably, if the confirmed histopathological diagnosis as gold standard, the sensitivity and specificity of the first postoperative histopathology were 93.10% and 98.55%, respectively (Table 3). The sensitivity of the first postoperative histopathology was lower than that of TG mRNA marker, but did not reach the statistical difference (93.10% vs. 98.85%, P = .07). However, the specificity of the first histopathology was higher than that of TG mRNA detection (98.55% vs. 95.65%, P < .001) (Table 3).
| Confirmed pathologic diagnosis | TG expression | TSHR expression | The first postoperative pathologic diagnosis | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Metastasis | Non-metastasis | |
| Metastasis | 86 | 1 | 74 | 13 | 81 | 6 |
| Non-metastasis | 3 | 66 | 5 | 64 | 1 | 68 |
| Sensitivity | 98.85% | 85.06% | 93.10% | |||
| Specificity | 95.65% | 92.75% | 98.55% | |||
| Accuracy | 97.44% | 88.46% | 95.51% | |||
| Positive predict value | 96.63% | 93.67% | 98.78% | |||
| Negative predict value | 98.51% | 83.12% | 91.89% | |||
| Youden index | 0.95 | 0.78 | 0.92 | |||
We then detected the TSHR expression using the same approach. Compared with the confirmed histopathologic diagnosis, the sensitivity and specificity of TSHR mRNA marker for metastatic lymph nodes in patients with PTC were 85.06% (74/87) and 92.75% (64/69), respectively (Table 3). Compared with the TG mRNA marker and the first postoperative histopathology, the sensitivity and specificity of TSHR mRNA marker were lower for diagnosing the metastatic lymph nodes in patients with PTC (sensitivity: TSHR vs. TG, 85.06% vs. 98.85%, P-Fisher > 0.05, TSHR vs. postoperative histopathology, 85.06% vs. 93.10%, P-Fisher > 0.05; specificity: TSHR vs. TG, 92.75% vs. 95.65%%, P-Fisher < 0.001, TSHR vs. postoperative histopathology, 92.75% vs. 98.55%%, P-Fisher > 0.05) (Table 3). The PPR of TG, TSHR mRNA marker and the first postoperative histopathology to diagnose the metastatic lymph nodes in patients with PTC were 96.63%, 93.67% and 98.78%, while the Youden's index of them were 0.95, 0.78 and 0.92, respectively (Table 3).
4 DISCUSSION
PTC is characterised by high differentiation and low malignancy, but studies have demonstrated that patients with metastatic lymph nodes had higher mortality risk, as well as lung metastasis, than those without lymph node metastasis.36 About 30%–80% patient with PTC have cervical lymph node metastasis at the time of diagnosis, with the neck region VI (central region) being the most common.37 According to the AJCC staging system and the ATA risk prediction system, the cervical lymph node metastasis was the best predictor for mortality and recurrence of patient with PTC.38 Moreover, it is important to know lymph nodes status because it is helpful for the postoperative management of the patient with PTC. However, the evaluation of the cervical lymph nodes status is a big challenge, especially at the preoperation. Ultrasonography (US) is currently the most useful method to detect cervical lymphadenopathy.39 Although US is an accurate tool in the identification of metastatic nodes,39 FNA is usually required to confirm the metastasis because reactive lymph node enlargement is common in the neck region.40 However, FNA cytology was highly accurate but produced false negative or nondiagnostic results in up to 20% of cystic, small, or highly vascular lymph nodes.41, 42 Thus, it is important to identify the high specific biomarker for evaluating the cervical lymph node metastasis.
TG restrictedly expressed in thyrocytes, therefore, it is tempting to presume measurement TG in nonthyroidal tissues will help to diagnose the persistence, recurrence, or metastasis of PTC.43 In fact, measuring the concentration of TG protein in the lymph node FNA washout (FNA-Tg) has been introduced to identifying the lymph node metastasis from patients with PTC as early as 30 years ago.43 A several studies have demonstrated that FNA-Tg measurement was useful to detect cervical lymph node metastasis and had high accuracy in diagnostic performance.44, 45 However, the sensitivity and specificity of FNA-Tg in detecting lymph node metastasis have been influenced by thyroid or TgAb status.44, 46 In a meta-analysis, Grani et al. reported that the FNA-Tg measurement had a high pooled sensitivity (96.9%) and specificity (94.1%) in the detection of cervical lymph node metastasis for patients with differentiated thyroid carcinoma (DTC) after thyroidectomy. However, the pooled sensitivity was only 86.2% and specificity was 90.2% for the DTC patients without thyroidectomy.44 More recently, Kim et al. reported that measurement of the FNA-Tg level increased preoperative diagnostic accuracy for the detection of metastatic lymph nodes in patients with PTC. They recommended that diagnostic accuracy was higher using a 20 ng/mL FNA-Tg cutoff level, but the results only exhibited 86.6% sensitivity, 66.7% specificity and 81.7% accuracy.35 Given that TG protein could release from thyrocytes to the cervical lymph node by drainage of lymphatic vessels, we presumed that measurement of the mRNA level in tissues of the neck lymph node might be a best marker to detect the cervical lymph node metastasis in patients with PTC. In this study, we detected the mRNA levels of TG and TSHR in the 156 FFPE samples of cervical lymph nodes from 89 patients with PTC by nest PCR, and found that the expression of TG as biomarker to diagnose the cervical lymph node metastasis in the patients with PTC showed a sensitivity of 98.85% and a specificity of 95.65%, with Youden's index up to 0.95, which had an excellent diagnostic accuracy for the detection of metastatic lymph nodes in patients with PTC when compared with the measurement of TG protein. However, measurement of the mRNA level of TSHR to detect the cervical lymph node metastasis in the patients with PTC showed relatively lower sensitivity (85.06%) and specificity (92.76%).
The postoperative histopathology has been considered as a gold standard to detect the lymph node metastasis in the patients with PTC. Interestingly, out of the 74 FFPE samples without lymph nodes metastasis based on the first postoperative histopathology, 9 samples have been detected the ectopic expression of the TG mRNA. Out of them, 6 samples have been reinterpreted as truly metastasis in the confirmed histopathology by two pathological experts, these results have also been verified by immunohistopathology using antibody to TG protein. These findings indicated that histopathology does not evaluate precisely the lymph nodes status in some patients with PTC. According to the recommends by American Society of Clinical Oncology (ASCO) or The College of American Pathologists (CAP), the standard approach for postoperative histopathology was followed by slicing the lymph nodes into 2 mm tissue blocks and placing it in an embedding cassette. Once these tissue blocks were embedded in paraffin, one section from the surface of each block was examined by microscopy to detect the lymph node metastasis.47 Thus, the current approach recommended by both CAP and ASCO was to make sure that we did not missed any metastasis larger than 2.0 mm, but metastases smaller than 2 mm will be missed.47 Notably, in the present work, the measurement the ectopic expression of the TG mRNA, a quantitative method has been used to evaluate the lymph nodes status. Thus, this method not only used to detect the lymph nodes’ metastases, but also to evaluate the tumour burden in lymph nodes. In fact, all of the 6 metastatic lymph nodes, which have been missed cancer metastasis at postoperative histopathology but TG mRNA tested positive, were micrometastasis. These findings indicated the measurement of ectopic expression of the TG mRNA cervical lymph node might be a best biomarker to evaluate the micrometastasis for patient with PTC.
This study has several limitations. First of all, the method for TG mRNA gauging was just used after prophylactic central neck dissection, but not in the preoperation to decide the best type of the surgical procedure. It will be investigated in the further once the samples have been recruited. Second, more lymph nodes should be studied and more clinical centres should be participated, although the measurement of the TG expression to detect the lymph node metastasis is an effective supplementation for conventional histopathological examination in this study.
To our knowledge, our study provided an excellent and effective diagnostic method to evaluate lymph node metastases for patient with PTC at the time of postoperation. The method should be referred to evaluate the lymph nodes status for patient with PTC, a strong support to the histopathological diagnosis.
AUTHOR CONTRIBUTIONS
Conceptualisation: Mei Dong and Huai-dong Song. Data curation: Wen-hua Du, Ying-chao Chen, Jing Wu, Feng-Yao Wu, Shuang-xia Zhao, and Bin Hai. Tissue sample preparation: Ning Jin, Yuan-yuan Zhang, and Bao-Lan Ji. Funding acquisition: Huai-dong Song. Methodology: Wen-hua Du, Ying-chao Chen, and Mei Dong. Data analysis: Wen-hua Du and Mei Dong. Writing—original draft preparation: Wen-hua Du. Writing—review and editing: Mei Dong and Huai-dong Song. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by National Science Foundation of China (Grant number: 82270826, H.D.S).
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
The study was conducted in accordance with the ethical principles of WMA Declaration of Helsinki and approved by the ethics boards of Linyi People's Hospital (202401-H-025).




