Roads diverged in a wood: Proteins and RNAs encoded by cytochrome b (CYTB) gene in health and disease
Cijie Du, Baodan Chen and Yile Huang contributed equally to this work.
Abstract
Background
The cytochrome b (CYTB) gene, a crucial component of the mitochondrial genome, plays a multifaceted role in cellular metabolism, energy production and various biological processes.
Main body
It is well known that the CYTB gene encodes a subunit of complex III in the electron transport chain, which is vital for the oxidative phosphorylation process and ATP generation. Various studies report that the CYTB gene not only encodes a core protein in the mitochondrial respiratory chain but also produces a long non-coding RNA called lncCYTB, which participates in a variety of physiological and pathological processes. Inspiringly, a study has recently revealed that the CYTB gene also encodes a novel 187 amino acid long polypeptide, CYTB-187AA, a mitochondrial DNA-encoded protein produced by cytosolic translation and important for early mammalian development.
Conclusion
This review will provide insight into the functional and expression properties of the CYTB gene, as well as its unique non-coding RNA signature, and describe the diseases associated with the CYTB gene, ranging from mitochondrial dysfunction to more complex genetic disorders.
1 INTRODUCTION
It is generally believed that the mitochondrial genome contains 37 genes, including 13 polypeptides which are the core subunits of the oxidative phosphorylation (OXPHOS) complexes, 22 transfer RNAs and two ribosomal RNAs, which are responsible for the transcription and translation of 13 polypeptides.1 The mitochondrial OXPHOS system is pivotal to eukaryotic energy metabolism, which is composed of four electron transport complexes embedded in the inner mitochondrial membrane. The four complexes of the electron transport chain including NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), cytochrome reductase (complex III) and cytochrome c oxidase (complex IV), generate a proton gradient in the inner mitochondrial membrane by oxidising the reducing equivalents entering the mitochondria, which subsequently drives the production of ATP through the dissipation of the proton gradient. It has been reported that the OXPHOS chain's components are multiprotein complexes assembled from subunits encoded in nuclear DNA and mitochondrial DNA (mtDNA).2
Among the four complexes, mammalian complex III consists of 10–11 subunits, of which, cytochrome b (CYTB) is a catalytic centre of this complex, the only subunit encoded by the mitochondrial genome.3 CYTB in complex III is involved in transmembrane electron transfer, by which redox energy is converted into proton motive force.4 In addition, the CYTB gene has also been verified to produce a high abundance of a long non-coding RNA (lncRNA), lncCYTB, an RNA retained in or exported from mitochondria to participate in various physiological and pathological processes.5, 6 A recent study has newly discovered that the CYTB gene also encodes a 187-amino-acid-long polypeptide, named CYTB-187AA, a mtDNA-encoded protein arising from cytosolic translation, which plays essential roles in early development in mammals.7 In this paper, we will delve into the functional and expressional aspects of the CYTB gene and introduce its unique non-coding RNA characteristics and the evolution of CYTB. Additionally, the intricate roles of the CYTB gene in cellular metabolism and its implications for mitochondrial health will be described. Finally, we discuss the diseases associated with the CYTB gene, extending from mitochondrial dysfunction to more complex genetic disorders.
2 THE PROTEINS ENCODED BY THE CYTB GENE
CYTB was first identified in 1922 and complex III was first isolated from bovine heart mitochondria in 1962,8 but the first crystal structure of bovine mitochondrial complex III was not published until 1997.9 The CYTB gene is located in the heavy strand of mtDNA and its mRNA encodes a polypeptide that is the only mtDNA-encoded subunit of complex III.10 Complex III couples the oxidation of ubiquinol and the reduction of cytochrome c to transfer electrons and concomitantly pumps four protons to the mitochondrial inner membrane space (IMS). This complex functions in a homodimeric structure and contains three catalytically active subunits: cytochrome b, cytochrome c1 (CYC1) and the Rieske iron‒sulphur protein (ISP). Cytochrome b (CYTB) is a transmembrane protein that includes two b-type hemes, heme bL and heme bH, which form the low-potential redox chain, whereas heme c1 in cytochrome c1 forms the high-potential redox chain along with iron‒sulphur clusters. There are two ubiquinone (CoQ) binding sites in CYTB distributed on either side of IMS, one of which is the ubiquinol (QH2) oxidation (Qo) site associated with cytochrome bL, which is located at the cytoplasmic side. The other is the Q-reduction (Qi) site related to cytochrome bH, which is on the side of the matrix.11 The mechanism of electron transfer in complex III is referred to as the Q-cycle. When the first QH2 binds to the cytoplasmic side, QH2 releases one electron to the Fe‒S cluster and is oxidised to ubisemiquinone (QH−) with the release of two protons into IMS at the Qo site, due to the higher potentials of Fe‒S cluster and heme c1 compared to heme b. The Fe‒S cluster transfers the electron to cytochrome c1 and in turn to cytochrome c, a lipid-soluble electron mobile carrier. Then, the QH− with strong reductive properties forms and rapidly donates the second electron to cytochrome bL at the Qo site, which would later be transferred to cytochrome bH at the Qi site and oxidised CoQ to QH−. To accomplish the Q-cycle, the second QH2 molecule is oxidised in the same way at the Qo site with two protons released and finally reduces the ubisemiquinone radical at Qi.11, 12 Most importantly, the key to the Q cycle is an electron bifurcation at heme bL, which results in the reduction of cytochrome c and quinone on separate sides of the complex.13 In summary, CYTB acts as an indispensable component of complex III (Figure 1).
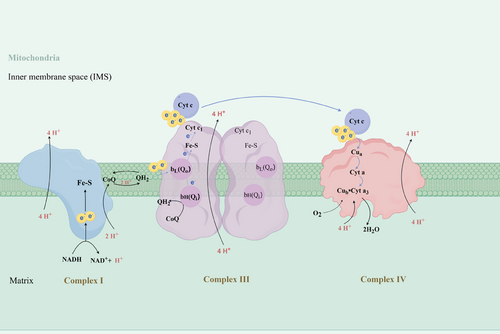
Evidence has accumulated to support the notion that CYTB is fundamental to the assembly and functions of complex III.14, 15 A recent study demonstrated that the CYTB carboxy-terminal region is required for the correct regulation of CYTB synthesis and respiratory growth, acting through an assembly-feedback loop that regulates COB mRNA translation.16 Furthermore, the c-terminal region of CYTB is indispensable for the regulation of complex III assembly, and its absence results in the accumulation of abnormal early subassembly of the bc1 complex. Due to the lack of formation of typical intermediates, complex III assembly is abrogated.16 Mutations in the CYTB gene cause complex III assembly disorders as well as mitochondrial diseases. Studies have shown that there is a structural dependence among complexes I and III, and that detrimental mutations in the CYTB gene lead to assembly disorders of complex III, which also affect the stability and activity of complex I.17, 18 Numerous patients with nonsense, missense or shifted-code mutations in the CYTB gene leading to mitochondrial disease have been described, with clinical manifestations ranging from mitochondrial encephalopathy,19, 20 cardiomyopathy21 and multisystemic disease,22 the vast majority of which are associated with severe exercise intolerance.23 It was found that the mitochondrial function of cardiomyocytes was reduced in PTCD2 (pentapeptide repeat structure and protein 2) gene-deficient mice, with substitute of ventricular cardiomyocytes by fibro-fatty tissue, suggesting that the dysfunction of this protein is associated with heart failure (HF). Northern blotting experiments show that PTCD2 deletion results in reduced CYTB mRNA transcripts in mouse heart, liver and skeletal muscle mitochondria, leading to a specific decreased enzymatic activity of complex III.24 Respiration-deficient phenotype and lipid-dependent catalytic activity are produced by mutating two residues of CYTB that are engaged in proton conductance for ubiquitin oxidation.25 Replacement of the conserved residue Tyr in CYTB with Cys not only leads to impaired electron transfer activity but also to increased reactive oxygen species production.26 Another study of blood from patients with hypertrophic cardiomyopathy shows that the p.C93Y mutation on the MT-CYB gene resulted in disruption of the tertiary structure of CYTB due to helical substitution, interfering with protein‒heme interactions.27
It is generally known that the mitochondrial genome encodes 13 proteins essential for oxidative phosphorylation.27 Inspiringly, Hu et al. have recently revealed a dual translation pattern of the mitochondrial gene CYTB and disclosed a novel protein containing 187 amino acids called CYTB-187AA, which is encoded by mtDNA while translated using the standard genetic code on the cytosolic ribosomes.7 CYTB-187AA was originally predicted using ORFfinder, which can search for open reading frames (ORFs) in a DNA sequence by using standard or other special genetic codons, and then rigorously validated by liquid chromatography‒tandem mass spectrometry with endogenous characteristic peptides and western blot. The MoonTag system is utilised to confirm the cytoplasmic translation of CYTB-187AA, using the standard genetic code rather than the mitochondrial genetic code, which suggests a new pattern of mtDNA-encoded protein arising from cytosolic translation (mPACT) (Figure 2). Furthermore, the important role of CYTB-187AA in early development is first identified in this study and the underlying mechanism is also disclosed. CYTB-187AA is mainly localised in the mitochondrial matrix and interacts with SLC25A3, a mitochondrial phosphate carrier that shuttles across the inner membrane for ATP production,28 to promote the primed-to-naive transition (PNT) process. CYTB-187AA and SLC25A3 are both higher expressed in ESCs than in EpiSCs and silence of CYTB-187AA results in decrease of the expression level of SLC25A3 in EpiSCs and suppresses the PNT process. Given that SLC25A3 is responsible for ATP synthesis by mediating the transport of mitochondrial phosphate across the inner membrane,29 they sought to delineate the specific role that ATP plays in the process of PNT by the CYTB-187AA. They found that ATP levels are higher in the naive stage and CYTB-187AA silencing reduces ATP levels in EpiSCs, which could be rescued by overexpression of SLC25A3, demonstrating that CYTB-187AA promotes PNT in an ATP-dependent manner through interacting with SLC25A3. Interestingly, although CYTB protein and CYTB-187AA are both involved in mitochondrial energy metabolism, they function in different ways, with the former producing ATP and the latter translocating ATP raw materials. In brief, CYTB protein acts as the constitutive protein in mitochondrial complex III to transfer electrons and functions in respiratory activities for constructive energy supply while CYTB-187AA regulates early development by modulating ATP production through SLC25A3 by transporting mitochondrial phosphate. Moreover, female mice have reduced numbers of ovarian follicles and decreased fertility after CYTB-187AA knockdown, further implicating an important role for CYTB-187AA in early mammalian development.7
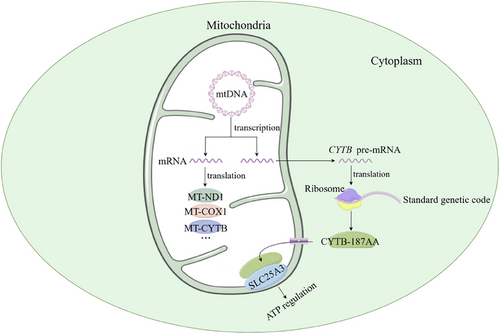
3 LONG NON-CODING RNA DERIVED FROM THE CYTB GENE
In a study published in 2011, Rackham et al. discovered a high expression level of lncRNA in the region of the mitochondrial genome complementary to the gene encoding CYTB mRNA by analysing strand-specific deep sequencing datasets, called lncCYTB. They confirmed the expression of lncCYTB by northern blotting and strand-specific qRT-PCR and revealed the abundance of lncCYTB RNAs is identified as 14% of its complementary coding CYTB mRNAs.6 As nuclear-encoded proteins can affect the expression of mitochondrial lncRNAs and thus its functions,24 lncCYTB is also found to be regulated by the nuclear-encoded mitochondrial proteins involved in RNA processing. Unlike CYTB mRNA, lncCYTB RNAs mainly form intermolecular double strands to protect themselves from RNase I cleavage. As complementary mRNA is in excess compared to lncCYTB RNAs and may have a protective role for lncRNA, lncCYTB is suggested to play a functional role in stabilising CYTB mRNA or regulating its expression. They further revealed that, like most lncRNAs, lncCYTB is cell and tissue-specific.6 A recent study has confirmed the interaction between lncCYTB and the mitochondrial genome, especially the CYTB region, that the hyperglycemic environment downregulates lncCYTB expression, contributing to the mitochondrial genomic instability and the downregulation of CYTB transcription. High glucose also diminishes the interaction between lncCYTB and mtDNA, thereby mtDNA becomes vulnerable to damage and the number of protective nucleoids is reduced. They further found that diabetic mice and human patients with diabetic retinopathy present decreased lncCYTB expression, reduced nucleoid number and increased mtDNA damage, implying that lncCYTB has a major role in maintaining mitochondrial genomic stability and prevention of lncCYTB downregulation could inhibit the development of diabetic retinopathy.5 In addition, Zhao et al. have revealed aberrant shuttling of lncCYTB between mitochondria and the nucleus in cancer cells. They utilised RNA-FISH staining and found lncCYTB is localised primarily in the mitochondria in normal liver HL7702 cells while detected in the nucleus of hepatoma HepG2 cells and much higher in the nucleus, which was further confirmed by cellular fractionation assay. Thus, they suggest lncCYTB may function as a messenger factor during the mitochondria-nuclear crosstalk and aberrant shuttling of lncCYTB may play a critical role in abnormal mitochondrial metabolism in cancer cells. As a mitochondrial-encoded RNA, lncCYTB is conventionally localised within the mitochondria. However, in cancer cells, it is not only present in the mitochondria but also in the nucleus. The divergence in its subcellular localisation may be linked to metabolic reprogramming or alterations in cellular functions. Such differences could influence mtDNA stability, mitochondrial metabolic dysfunction, oxidative stress responses, cell cycle regulation and other signalling pathways, facilitating the interaction between lncCYTB in the mitochondria and the nucleus. Intriguingly, it appears that the epithelial‒mesenchymal transition does not impact the subcellular localisation of lncCYTB. Further investigations are warranted to ascertain whether the aberrant localisation of lncCYTB is associated with the malignant traits of cancer cells.30 Additionally, lncCYTB is also capable of functioning within the cytoplasm. Chen et al. found that lncCYTB is reduced during HF and overexpression of lncCYTB in the cytosol is found to improve cardiac function and affect the progression of HF.31 The underlying mechanism is disclosed that lncCYTB acts as a competitive endogenous RNA to sponge miR-103-3p and thereby inhibits cardiomyocyte hypertrophy and isoprenaline-mediated reactive oxygen species (ROS) activation through the miR-103-3p/PTEN axis, consequently ameliorating transverse aortic constriction-induced cardiac dysfunction. Furthermore, a circulating lncRNA named LIPCAR can predict survival in HF patients,32 and its 5′ half is wholly contained within the mitochondrial lncCYTB gene.6
4 EVOLUTION OF CYTB AND COMPARATIVE ANALYSIS BETWEEN MITOCHONDRIA AND CHLOROPLASTS
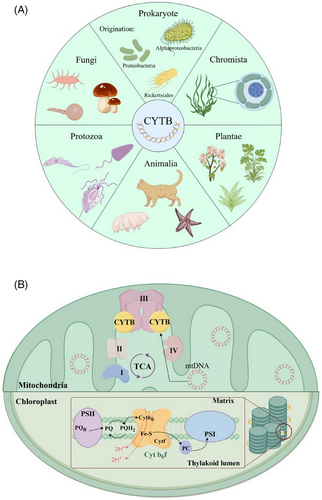
Exploring the evolutionary relationship between species is still a tricky problem. Historically, taxonomic delineation and phylogenetic inference have relied on fossil records and phenotypic traits, which while informative, are particularly challenging in some cases. Traditional identification methods, necessitating dissection to examine internal morphological features, are laborious, time consuming and require specialised expertise. With the emergence of molecular biology, phylogenetic analyses have been revolutionised through the construction of phylogenetic trees based on molecular data.32 However, inconsistencies in phylogenetic interpretations arise from the use of different genetic markers, such as the D-loop and ND4 sequences compared to the CYTB gene.33 The CYTB gene, due to its high variability that enables the differentiation of closely related species and its conservation sufficient to delineate relationships at higher taxonomic levels, has become an increasingly favoured genetic marker for evolutionary studies. The endosymbiotic origin of mitochondria that contains the CYTB gene is a defining event in the evolution of eukaryotic cells. Strong phylogenetic evidence supports the eukaryotic CYTB genes likely originated from an ancient prokaryotic gene in Alphaproteobacteria based on shared conserved domains,34, 35 and most studies report the idea that it evolves from an ancestor related to Rickettsiales,36, 37 while another study suggests that it evolves from a proteobacterial lineage that branched off before the divergence of all the Alphaproteobacteria sampled38 (Figure 3A). The cytochrome b (CYTB) amino acid sequences contain six conserved domains, including heme bL binding sites, heme bH binding sites, Qo binding sites, Qi binding sites, the interchain domain interface, and the intrachain domain interface, in all five kingdoms of eukaryotes as well as the prokaryotes (Figure 3A), which indicates that the CYTB gene evolved slowly; maintained similar biological function and diverged from a common ancestor.34
Thus, the CYTB gene is widely used in the construction of phylogenetic trees. For instance, the mitochondrial CYTB gene has been proposed as a novel and robust marker for the diagnosis and phylogenetic analysis of Prototheca algae species. A rapid and direct PCR-restriction fragment length polymorphism assay, based on species-specific polymorphisms within the CYTB gene, has been developed to identify and distinguish all currently described Prototheca species.39 The application of the CYTB gene in phylogenetic analyses has provided molecular evidence supporting the classification and phylogeny of murinae species, as demonstrated by the Zou team's study. This contributes significantly to the understanding of the evolutionary processes and differentiation mechanisms within Myospalacinae.40 Accurate identification of phlebotomine sandfly species is very important for the prevention of transmitted diseases. Analysis of 67 CYTB sequence differences of six phlebotomine individuals showed that the intra-specific genetic distance of the CYTB gene was smaller than the inter-specific genetic distance, and the CYTB gene could be used as an effective molecular marker for the identification of phlebotomine sandfly in China. Neighbour-joining (NJ) phylogenetic trees of six sandfly species in China were constructed based on the mtDNA CYTB sequence. The NJ tree also showed two branches, similar to the NJ tree based on the cytochrome c oxidase subunit I gene (COI) sequence. Therefore, the gene CYTB serves as a robust DNA barcode for sandfly, enabling the effective differentiation of the intraspecies and interspecies genetic distances. This approach offers a novel avenue for advancing the study of sandfly taxonomy.32 Comparative genomics revealed a high degree of conservation of CYTB in different species and highlighted its important role in oxidative phosphorylation. The CYTB gene has undergone various mutations and selective pressures throughout evolution, reflecting the metabolic needs of organisms and environmental adaptations, exemplified by the accelerated evolutionary rate observed in sea turtles, which may be attributed to their more active lifestyle.41
CYTB is an essential component of the electron transport chain in both mitochondrial cytochrome bc1 and chloroplast cytochrome b6f complexes. Despite the differences in primary sequences, mitochondrial and chloroplast CYTB exhibit remarkable similarities in tertiary structures, transmembrane topologies and functional roles (Figure 3B). Notably, while mitochondrial CYTB is encoded by a single gene (CYTB), its chloroplast counterpart is encoded by two separate genes: petB and petD, which produce Cytb6 and subunit IV, respectively.42 These chloroplast proteins are partially homologous to the N- and C-terminal regions of mitochondria CYTB.43 The structural conservation between mitochondrial and chloroplast CYTB is particularly striking. Chloroplast Cytb6 appears to possess five spanning domains analogous to the first five domains of mitochondrial CYTB, while subunit IV of the chloroplast cytochrome b6f complex likely incorporates functions similar to those of the COOH-terminal region of mitochondrial CYTB.44-46 Mitochondrial CYTB in the bc1 complex participates in ATP synthesis by facilitating electron transfer from ubiquinone (CoQ) to the cytochrome c reductase. Similarly, the chloroplast cytochrome b6f complex, also known as plastoquinol-plastocyanin reductase, mediates electron transfer from plastoquinol (QH2) to plastocyanin (Pc) within the thylakoid membrane. Although the directionality of electron flow differs between bc1 and b6f complexes, the electron transfer reactions in both systems are functionally homologous.47 These processes drive proton translocation across their respective membranes, ultimately powering ATP synthesis via ATP synthase. The structural similarity extends to the catalytic Qo quinone oxidation site, a critical component of the Q cycle, which is highly conserved between mitochondrial cytochrome bc1 and chloroplast cytochrome b6f complexes.48 This conservation, along with the numerous functional parallels between mitochondria CYTB and chloroplast Cytb6, suggests a potential common ancestral role in ATP-dependent energy production.49 Thus, comparative analysis of mitochondrial and chloroplast CYTB gene provides valuable insights into cellular respiration and photosynthesis. Furthermore, it enhances our understanding of species’ evolutionary relationships and elucidates the functional divergence of this gene across different organelles.
5 CYTB GENE IN HEALTH AND DISEASE
As a very important polypeptide, mitochondrial cytochrome b could promote energy metabolism by oxidative phosphorylation. Several studies have shown that CYTB plays an important role in animal reproduction because its features are easy to affect the animal's physiological function, especially for the energy delivery and supply of female germ cells.50 Our study published in 2024 showed mitochondria are capable of utilising the cytoplasmic standard genetic code to synthesise a novel functional protein CYTB-187AA, which is expressed across a spectrum of tissues, and the research revealed that the knockout of CYTB-187AA primarily impacts female fertility, significantly reducing female mice's reproductive capacity. Subsequent histopathological examination of the ovaries from CYTB-187AA knockout mice reveals no alteration in the ratios of primordial, growing, mature and atretic follicles. However, the knockout mice exhibited reduced fertility due to a concomitant decrease in the overall number of ovarian follicles.7 Before this was reported, the CYTB gene was used in pig breeding on the influence in females and the role of porcine reproductive, and they found that CYTB mutation affects the litter size of the sow and mortality in young pigs.50 What's more, the level of CYTB was observed to escalate approximately threefold as follicles transitioned from an immature to an active steroidogenic state, corpus luteum stage, in comparison with the cytochrome concentration within the mitochondria of both immature and superovulatory rats.51 Thus, the CYTB gene may be used as a marker for studying female reproduction in the future.
Abnormalities in the CYTB gene have been identified to be closely associated with the onset and progression of various human diseases (Figure 4). Dysfunction of mitochondria is known to be linked with diabetes complications. A study in 2023 has provided evidence for the interaction between lncCYTB and CYTB, suggesting that the reduction in CYTB gene transcription can adversely affect diabetic retinopathy. The research team utilised human retinal endothelial cells as their model and exposed these to high-sugar culture conditions. Their findings revealed that lncCYTB was capable of downregulating the transcription of CYTB under hyperglycemic conditions, thereby inhibiting the activity of complex III and compromising mitochondrial function.5 Adipose tissue from patients with fatty liver disease exhibited significant variations in the CYTB gene, and subsequent CYTB gene sequencing analysis revealed that these variations were correlated with histological features, including the degree of fat degeneration, lobular inflammation, necrosis and fibrosis. Furthermore, studies on obese and morbidly obese individuals have uncovered phenotypic traits genetically associated with the CYTB gene, indicating that the number of CYTB variants is inversely proportional to the body mass index.52 In 2019, the emergence of coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), posed a significant public health threat globally. Mitochondria have been identified as a key target for SARS-CoV-2 infection, with oxidative stress being a contributing factor to mtDNA damage in patients with COVID-19. A pilot study was conducted to investigate the correlation between mtDNA mutations and clinical outcomes, analysing DNA sequences from blood samples of 65 confirmed COVID-19 patients. The study revealed 16 distinct mutations within the CYTB gene, characterised by a notable frequency of missense and synonymous mutations. However, no significant correlation was found between CYTB gene mutations and patient age or biochemical indices. The high prevalence of CYTB mutations in COVID-19 patients underscores the pivotal role of this gene in the disease's pathogenesis, highlighting the importance of genetic background in disease outcome and severity as determined by clinical and laboratory assessments.53, 54 To delve deeper into the molecular underpinnings of respiratory dysfunction associated with CYTB gene mutations, researchers on the Fisher team introduced six specific mutations into the yeast CYTB gene. These mutations were found to severely compromise the structural integrity and functional efficacy of the bc1 complex, thereby adversely affecting cellular respiratory processes. Among these, mutants G33S, S152P, G291D and ISP252-259 were identified as posing a high risk.15 Five of six renal excision method was used to construct a chronic kidney diseases (CKD) model in rats, finding that CKD of rat intestinal duodenal cytochrome b (Dcyt-b) and divalent metal from 1 (DMT) expression is impaired, concurrent with reduced serum iron levels. These results suggest that DCYTB and DMT-1 are associated with decreased iron intake in CKD patients.55 Prior research from the same investigative team has observed a similar phenomenon in rats with HF.56 The interplay between HF, CKD and anaemia is referred to as cardiorenal anaemia syndrome.57 These intestinal iron transporters are expected to become prospective therapeutic targets for cardiorenal anaemia syndrome.
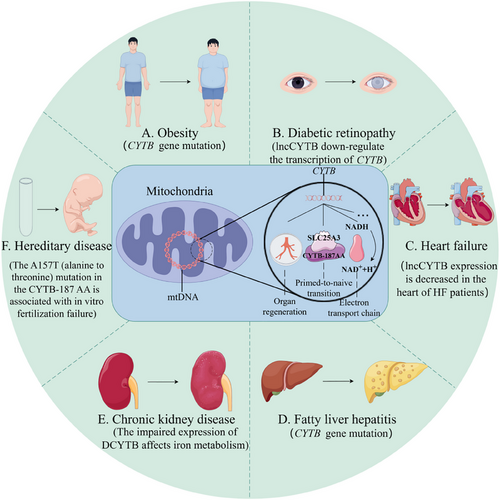
In addition to a substantial body of research on cytochrome b in mammals, there has been progress in the study of the CYTB gene in other animal species. Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, is an infectious illness also known as American trypanosomiasis, affecting approximately 6–7 million people globally, predominantly in Latin America.58, 59 To address the issues of high toxicity and high treatment failure rates associated with current therapies, a novel T. cruzi inhibitor, GNF7686, has been developed. This inhibitor targets the cytochrome b of T. cruzi, thereby retarding its growth and holding promise as a novel clinical therapy for Chagas disease.60 Furthermore, studies have shown that freshwater eels exhibit an increase in ovarian mitochondrial cytochrome b expression, with transcript levels progressively rising from the early to late stages of vitellogenesis. This suggests that elevated levels of cytochrome b transcripts facilitate enhanced ATP synthesis during specific phases of oogenesis or early zygote development.61
6 CONCLUSION AND PERSPECTIVES
Cytochrome b gene is integral to mitochondrial function, encoding two proteins CYTB and CYTB-187AA in a dual translation pattern that CYTB constitutes a pivotal component of the electron transport chain and CYTB-187AA modulates ATP production through SLC25A3. The tertiary structure of CYTB transmembrane helices is characterised by a high degree of delicacy and complexity,4 which is essential for its role in the generation of ATP and overall cellular energy metabolism. CYTB-187AA regulates early development by modulating ATP production by interacting with SLC25A3 by transporting mitochondrial phosphate.7 In addition, the CYTB gene also encodes a lncRNA lncCYTB that plays pivotal roles in important biological processes and human diseases.5, 30, 31 Therefore, mutations within the CYTB gene can precipitate mitochondrial dysfunction, thereby implicating a spectrum of health issues. The CYTB gene is susceptible to mutations, deletions or substitutions that can impact the reproductive system's functionality. Given the potential implications of the CYTB variations on reproductive health, the genetic profiling of this gene could serve as a valuable biomarker for preconception screening initiatives. Future research should encompass comprehensive sequencing of the entire mitochondrial genome, coupled with extensive association studies focusing on a broader array of reproductive phenotypes, which not only can deepen our understanding of the genetic underpinnings of reproductive health but also inform the development of targeted interventions and preventive strategies.
Due to its conservation at higher taxonomic levels while variability between closely related species, the cytochrome b gene is utilised for elucidating the phylogenetic relationships among organisms. Through comparative analysis of 20 mammalian gene sequences, the evolutionary dynamics, including the rate and pattern of CYTB gene evolution, as well as the phylogenetic utility of substitution, silencing, switching and translational changes in this gene have been progressively elucidated.62 The gene sequence variability positions it as an invaluable asset in systematic biology, capable of resolving taxonomic discrepancies across multiple hierarchical levels.50 In conjunction with mitochondrial ribosomal DNA (rDNA), the CYTB gene has been instrumental in addressing profound phylogenetic inquiries, exemplified by its contribution to understanding the evolutionary origins of tetrapods.63 The CYTB gene, a phylogenetic probe, in comparison to mitochondrial rDNA or non-coding sequences, offers a more accessible avenue for comparative analyses of protein-coding sequences across mammalian lineages.62 Furthermore, the investigation into CYTB mutations has propelled the advancement of CYTB protein models, thereby bridging gaps in the knowledge of functional domains, conserved regions and inhibitory sites associated with the two reaction centres, Qo and Qi. This research is not only pivotal for the comprehension of the molecular mechanisms underpinning electron transport but also significantly contributes to the study of drug resistance.64 The exploration of the structure‒function relationship of this protein provides deeper insights into the utility of evolutionary studies of the CYTB gene.
The CYTB gene is recognised for its multifaceted involvement in a spectrum of biological processes, encompassing animal organ regeneration, mitochondrial electron transport, and adaptive responses to extreme environmental conditions, as well as responses to cadmium, calcium and copper ions.65 Moreover, there are different degrees of research progress to the CYTB gene in the fields of biology, botany, microbiology and parasitology. The cytochrome b further research can deepen our understanding of breathing and the role of energy production in cells, such as the development of targeted cytochrome b mutation treatment interventions, to treatment of mitochondrial disease. Mitochondrial diseases still plague human groups of different ages. At present, mitochondrial replacement therapy or the commonly known concept of the three-parent baby can correct the reproductive problems caused by mitochondrial defects, which can fundamentally prevent severe mitochondrial genetic diseases.66 The CRISPR/Cas9 technology enables the targeted deletion of specific sequences within mutant DNA. On this foundation, the optimised mito-Cas9 system can directly edit mtDNA, which may hold therapeutic potential for treating mitochondrial diseases caused by mutated mtDNA.67 Last but not least, the elucidation of potential interactions between CYTB and other proteins and enzymes within the electron transport chain remains an area for exploration. The sustained study of cytochrome b is anticipated to yield novel insights into the treatment of mitochondrial diseases and to pave the way for innovative strategies aimed at ameliorating human health.
AUTHOR CONTRIBUTIONS
Cijie Du, Baodan Chen and Yile Huang drafted the manuscript. Xingguo Liu and Ying Hua Su supervised the study. All authors critically reviewed and approved the manuscript.
ACKNOWLEDGEMENTS
This work was financially supported by the National Key Research and Development Program of China (2023YFE0210100), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0480000), the National Natural Science Foundation projects of China (32025010, 32488301, 92254301, 92357302, 92157202, 32241002, 32261160376, 31970709, 32070729, 32100619, 32170747, 32322022, 32370782, 32371007, 32300608, 32300620 and 32471358, 32461160288, 32270377), the NSFC/RGC Joint Grant Scheme 2022/2023 (N_CUHK 428/22), the National Key Research and Development Program of China (2024YFA0916400, 2022YFA1103800, 2022YFE0210100, 2019YFA0904500, 2022YFF1002902), Major Project of Guangzhou National Laboratory (GZNL2024A03006, GZNL2024B01003), the Key Research Program, CAS (ZDBS-ZRKJZ-TLC003), the International Cooperation Program, CAS (154144KYSB20200006), the CAS Project for Young Scientists in Basic Research (YSBR-075), the Guangdong Province Science and Technology Program (2023B0303000023, 2023B1111050005, 2023A1515030231, 2022A1515110493, 2023B1212060050, 2021A1515012513, 2021B1515020096, 2022A1515012616, 2022A1515110951, 2023B1212120009, 2024A1515010782, 2024B1515040020, 2024A1515030120 and 2023TQ07A024), the Guangzhou Science and Technology Program (202102021037, 202102020827, 202102080066, 202206060002 and 2023A04J0414), Health@InnoHK funding support from the Innovation Technology Commission of the Hong Kong SAR, CAS Youth Innovation Promotion Association (Y.W. and K.C) and Basic Research Project of Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
Not applicable.




