Recent advances in mitochondrial replacement therapy and its future expectations
Abstract
The prevention of mitochondrial diseases is particularly important due to the lack of specific therapies. Therefore, mitochondrial replacement therapy (MRT) is expected to be a technology to prevent mitochondrial diseases. Admittedly, this technology sparked a lot of controversy and discussion. In this article, we review the recent advances in MRT, discuss its safety and ethical issues, and finally explore its potential to completely block the inheritance of mitochondrial diseases.
Seven years have passed since our report “Live Birth from Oocyte Spindle Transfer to Prevent Mitochondrial Disease.”1 Similar to the first birth after in vitro fertilization, the first birth following mitochondrial replacement therapy (MRT) ignited intense medical, ethical, and legal debates.2 However, the application of MRT technology to treat infertility due to oocyte-derived factors last year (2023) generated little debate,3, 4 with only minor ethical concerns. Nonetheless, we aim to clarify some public misunderstandings about MRT and enhance the education of genetic counselors.5
Currently, the public still refers MRT as “three-parent IVF.” However, it is crucial to recognize that the so-called third parent does not introduce new genetic traits. MRT primarily comprises four techniques: spindle transfer (ST), pronucleus transfer (PNT), polar body 1 transfer (PB1T) and polar body 2 transfer (PB2T). The primary distinction among these techniques is the variation in the genetic material transferred. In the case of ST, the spindle-chromosome complex from the mother's oocyte is transferred to the enucleated donor oocyte with healthy mitochondria. PNT involves fertilizing the oocyte from mother and donor synchronously, and then transferring the pronucleus from the fertilized mother zygote to a healthy fertilized but enucleated donor oocyte. PB1T utilizes the mother's first polar body (PB1) instead of the spindle, transferring PB1 to the enucleated donor oocyte with healthy mitochondria. PB2T uses maternal second polar body (PB2) in place of the female pronucleus, transferring PB2 to a healthy donor oocyte (Figure 1). The donated mitochondrial DNA (mtDNA) is similar to the replaced mtDNA in both nucleotide sequence and gene function, and in fact, mitochondria contribute only a small amount of DNA (approximate 0.1%)6; therefore, the donor should not be considered as a relevant third parent. Nevertheless, due to the involvement of nuclear transfer in MRT, it is often mistakenly equated with cloning technology. However, the essence of cloning is not the technical operation but rather the complete duplication and reprogramming of somatic cell nuclear. MRT involves only the haploid gamete genome transfer of the oocyte derived from the patient, not the nuclear transfer of somatic cells, and does not involve gene expression reprogramming. Additionally, mitochondrial replacement does not introduce new genes or DNA modification; it simply replaces an abnormal but otherwise similar set of mtDNA with a normal, naturally existing set. Therefore, it is completely incorrect to consider mitochondrial replacement as genetic modification or editing. The clinical application of MRT has been legislatively approved in the United Kingdom and in Australia in 2015 and 2022, respectively,7, 8 suggesting that MRT technology is considered ethically acceptable.
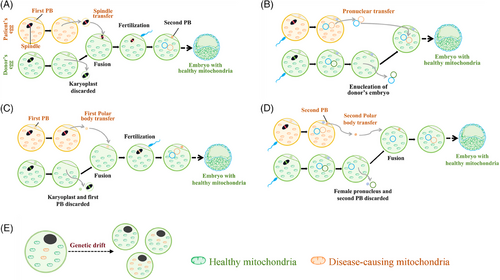
Nonetheless, a critical concern associated with MRT is the potential secondary risks posed to offspring resulting from nuclear transfer.9 At present, in MRT mice, no significant drift of mtDNA has been found in the offspring of MRT mice after 10 generation.10 The first monkey born via spindle MRT transfer is now 15 years old and has successfully produced offspring.11 Similarly, the first human baby born through spindle MRT transfer is now eight years old, and no major problems have been reported so far.1 Currently, no significant health issues have been found in infants born through infertility treatments utilizing MRT,3 although a long-standing concern remains regarding the probability of maternal mtDNA drift and even reversal after MRT,12 which could still pose a disease risk as a small number of maternally pathogenic mitochondria are still carried after MRT. In a recent report, among six infants conceived via MRT, one exhibited a maternal mtDNA drift from 0.8% at the blastocyst stage to 60% postnatally. Fortunately, the maternal mtDNA was normal and mutation-free. This phenomenon of drastic drift has not yet been convincingly explained,12 making it almost impossible to formulate effective measures to absolutely prevent drift. Nonetheless, what will be the situation if the initial maternal mtDNA carryover rate were further reduced? For example, reducing the initial carryover rate from < 1% might lower the risk of such drastic drift. Is it possible to completely avoid mtDNA drift or inversion by reducing the initial carryover rate to less than 1%? Technically, reducing the carryover rate is feasible. In 2023, Lyu's team from China reported innovative and improved spindle MRT techniques and second polar body MRT techniques,13, 14 which can mechanically remove the maternal cytoplasm around the spindle without causing membrane damage or perform a second cytoplasm removal during second polar body transfer. These techniques reduce the initial maternal mtDNA carryover level significantly compared to traditional methods, reducing interference and lowering the risks of chromosomal instability and DNA damage associated with traditional procedures.13, 14 The principle is to minimize the carryover into the reconstructed oocyte/fertilized zygote, so that even if drift occurs later, it does not result in a high proportion of heterogeneity and disease. However, long-term safety is still not fully guaranteed. Sha's team, also from China, reduced the mtDNA carryover to an undetectable level through the first polar body transfer, which is expected to completely block the inheritance of mutant mtDNA.9 It has been confirmed that PB1T/PB2T carries significantly fewer mtDNA copies than PNT/ST.15 However, why is mtDNA completely undetected in PB1T in reconstructed offspring mice? Is the mtDNA in PB1 potentially eliminated, and is its ultimate fate to be eliminated? The underlying mechanism needs further exploration. Undoubtedly, the optimal application of such technologies in human embryos, such as the optimal time for nuclear transfer, and the heteroplasmy variation of the resulting embryos and embryonic stem cells during development needs further exploration. Therefore, reducing or even eliminating the risk of mtDNA drift by reducing the initial carryover rate fully deserves further investigation in patient embryos to verify its safety and efficacy and prepare for its clinical application.16
Moreover, latent risks caused by MRT operations, such as epigenetic variations are yet to be explored. Although it has been shown that DNA methylation of PBNT does not alter considerably,17 more epigenetic analysis and assessment are required. Delayed DNA demethylation in the trophectoderm cells of human blastocysts obtained via MRT was reported recently.18 The mechanisms and risk levels of this phenomenon, along with corresponding optimization techniques, are not fully understood. While ensuring the safety of MRT operations, its clinical efficacy still needs cautious but necessary exploration. We recommended to prioritize clinical trials of MRT for Leber's hereditary optic neuropathy (LHON). LHON is caused by mitochondrial mutations and primarily affects the optic nerve and usually requires almost homoplasmy mutation to cause vision decline. We also recommended that the initial clinical trial be limited to transferring male embryos to minimize the genetic risk of passing on mutant mtDNA to the offspring.19 In this way, the clinical application of MRT will be able to ensure both the safety and efficacy. In addition, it provides deeper insights into the mechanisms of mtDNA drift and threshold effects. Other mutation sites with high onset thresholds, such as Leigh syndrome caused by m.9185 T > C (onset threshold of 100%), may also be considered to gradually extend the application scope of MRT. However, the diseases with a low threshold of pathogenic mitochondria, such as MELAS should be delayed until we have further information from the initial clinical trials. Hence, we would not recommend the initial clinical application of MRT to those conditions with a low threshold.
In summary, we assert that MRT will likely yield significant advancements in human fertility health, akin to the impact of In Vitro Fertilization (IVF), notwithstanding certain challenges.
AUTHOR CONTRIBUTIONS
Taosheng Huang conceived the idea and edited the manuscript. Qifeng Lyu contributed to the preparation and writing of the manuscript. Weiwei Zou was responsible for the design of the figure and editing of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This research was funded by the National Key R&D Program of China, grant number 2021YFC2700901.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
No data are generated in this review.




