Liquid biopsy—A biomarker-based revolutionising technique in cancer therapy
Subham Sarkar and Samraggi Chakraborty contributed equally to this article.
Abstract
Background and Aims
Cancer has grabbed the attention of scientists and medical professionals all over the world much more than any other disease. In the past few decades, the medical field has improved quite a lot but progress in the path to find a solution for cancer is very less. As the popularity of invasive technologies is diminishing in cancer treatment, scientists have come up withminimally invasive or non-invasive alternatives, among which liquid biopsy, by far is the most suitable.
Methods
Liquid biopsy is used to analyse nucleic acids, subcellular components and circulating tumour cells in various biological fluids for diagnosis of cancer. It can also be used to know the efficacy of cancer drugs in a patient by analysing multiple samples.
Outcomes
Liquid biopsy is becoming standard of care as it allows biopsy of those samples in which solid tumour biopsies are not possible. The diversity of sampling procedures, such as collection of urine for urothelial carcinoma or bladder or prostate cancer and phlebotomy for other types of cancer, make liquid biopsy one of the best methods for diagnosis of cancer.
Conclusion
This review aims in discussing the several techniques used for the detection of cancer biomarkers and some clinical manifestations due to the changes in the biomarkers which are analysed by liquid biopsy.
1 INTRODUCTION
Understanding the mechanisms of biomarkers in cancer diagnosis is crucial for improving patient outcomes and advancing personalised medicine. Biomarkers are molecular indicators that can be measured in biological samples such as blood, tissue or urine, and they play a fundamental role in the detection, diagnosis and management of cancer. Biomarkers can provide valuable information about the presence of cancer, its subtype and its molecular characteristics, which can guide treatment decisions.1 By elucidating the mechanisms underlying biomarker expression and function, technologies can help to improve the accuracy and reliability of diagnostic tests, reducing the risk of misdiagnosis and ensuring that patients receive the most effective therapies. Technologies that elucidate biomarker mechanisms can contribute to the development of novel diagnostic tools and therapeutic interventions. By uncovering the molecular pathways involved in cancer progression and metastasis, researchers can identify new targets for drug development and design innovative treatment strategies. By studying how biomarkers interact with cellular pathways and the tumour microenvironment, scientists can gain insights into the underlying causes of cancer and identify new biomarkers for early detection and risk assessment. There are different technologies that help improve or promote understanding of mechanisms of biomarkers.1 By harnessing these technologies and interdisciplinary approaches, researchers and clinicians can gain deeper insights into the mechanisms of biomarker action, interactions and disease pathogenesis, leading to more accurate biopsy results, early detection of disease and personalised treatment interventions for improved patient outcomes.
2 TECHNOLOGIES FOR DETECTION OF CANCER BIOMARKERS
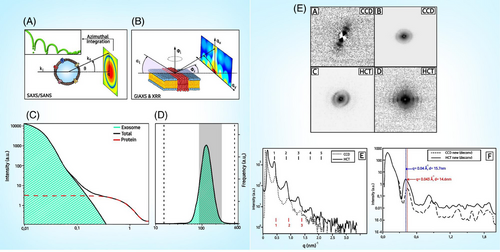
Sarhadi and Armengol in 2022 have explained in their paper that certain genetic variants increase cancer risk, classified by frequency and penetrance. BRCA1 and BRCA2 variants are linked to breast and ovarian cancer. Lynch syndrome variants have higher penetrance for colorectal cancer (CRC). Next-generation sequencing (NGS) detects pathogenic variants, but may miss some. Germline markers help to identify cancer susceptibility and predict therapy outcomes. Somatic mutations, common in cancer, vary in type and frequency among tumours. Transcriptomic alterations classify tumours into subtypes, aiding treatment prediction. Non-coding RNA, like miRNAs, plays roles in cancer development and metastasis.1 Epigenetic changes, including DNA methylation, are early events in carcinogenesis. Proteomic changes, including protein glycosylation, offer potential biomarkers. Cellular phenotype alterations, like DNA repair capacity and oxidative stress, reflect cancer progression and response to therapy. Cancer biomarkers are analysed from various samples, including tumour tissue and liquid biopsies like blood, urine, stool and exhaled breath. Different blood components, like white blood cells (WBCs) and circulating tumour cells (CTCs), are used for genetic variant detection and diagnosis of haematological cancers.1 Plasma and serum are crucial for detecting tumour-associated DNA, RNA and proteins. Urine is mainly utilised for bladder and prostate cancer testing, while stool samples are vital for CRC screening. Additionally, saliva, exhaled breath and extracellular vesicles (EVs) offer promising avenues for cancer biomarker discovery, although EVs' heterogeneity poses challenges. Exosomes are EVs, which represent the phenotypic characters of the cells where they are generated. Their count is the highest in cancer patients ranging to around 3800 trillion in the biological fluids. Exosomes have a dual nature in tumour progression and were shown to possess anti-tumour functions to prevent disease progression. Like other subcellular components, exosomes also have lipid bilayer, proteins and nucleic acids like DNA, RNA, and so forth, which act as important biomarkers. Exosomes contain a variety of proteins including enzymes, signalling molecules and structural proteins. Tumour-derived exosomes often carry specific oncoproteins, which can serve as biomarkers for cancer diagnosis and prognosis. The nucleic acids in exosomes are indicators of the genetic and epigenetic landscape of the originating tumour cells. The most common nucleic acid types included involve miRNAs and long non-coding RNAs (lncRNAs). miRNAs are mainly short length RNA species, which play a major role in cancer progression by interacting with the tumour-to-stromal interactions, immune invasion, angiogenesis and the tumour microenvironment. They get associated with the exosomes in the first place after being shredded from tissue damage or programmed cell death. A tested miRNA panel consisting of miR-21 and miR-1246 has been successfully combined with a few others to improve diagnostic efficiency for various CRC stages. LncRNAs are non-protein encoding RNA transcripts, usually longer than 200 nucleotides and are sometimes termed as ‘mRNA-like’ because of their plausibility to acquire modifications. They are capable of facilitating cell-to-cell communication to improve chemoresistance. Studies have proven to their role as minimally invasive biomarkers for gastrointestinal (GI) cancers mainly. Lipid composition of exosomes can also be indicative of their cellular origin and pathological state, providing another layer of potential biomarkers. As a result of being continuously released by cells exosomes can offer real-time information about tumour state and response to therapy allowing dynamic monitoring. Exosomal role in understanding tumour microenvironment is crucial due to their role in cell-to-cell communication, ability to modulate immune responses and influence metastasis. Determining changes at the biomarker level includes a variety of techniques that can analyse biological samples.1 All of them provide information contributing to comprehensive understanding of disease mechanisms, diagnosis, prognosis and treatment strategies. The various methods to detect changes in cancer biomarkers are enumerated in Table 1. The potential role of X-ray scattering experiments in exosome-based liquid biopsy (LB) is depicted in Figure 1. X-ray-based scattering experiments, such as X-ray diffraction and small angle X-ray scattering (SAXS), play a crucial role in the study of exosome-based LB for several reasons: these techniques allow for detailed analysis of the exosomal morphology. SAXS, in particular, can provide information on the size, shape and distribution of the exosomes without need for extensive sample preparation. X-ray scattering can help identify the compositional elements and molecular makeup of exosomes. X-ray scattering can be used to monitor the uniformity and quality of exosome preparations. At the end of X-ray-based scattering experiments the samples remain intact and can be used. Therefore, scattering of X-rays can be used to understand the composition and organisation of the lipid bilayer of the EVs and how they interact with different objects, which are some nanometres in size. These EVs can be used to deliver drugs to a target and have opened a new frontier in cancer therapy.
| Name of technique | Description | References |
|---|---|---|
| Polymerase chain reaction (PCR) |
This sensitive technique involves a reaction that amplifies specific DNA sequences. This helps in detection of genetic mutations, gene expression changes and presence of foreign DNA. Specific primers are annealed to single strands of DNA after the duplex has been denatured at a high temperature, followed by synthesis of new strands of DNA using the primers. The synthesis is allowed to happen for around 30 cycles. Real-time PCR helps in more accurate quantitation and cycle-wise detection of DNA synthesis using dyes and probes. |
2 |
| Next-generation sequencing (NGS) | It provides comprehensive genomic information used to identify epigenetic changes and gene expression profiles. NGS technologies enable the comprehensive analysis of genetic variations, including mutations, gene expression profiles and epigenetic modifications. By sequencing tumour DNA, RNA and other biomolecules, NGS can provide insights into the molecular mechanisms underlying cancer development, progression and response to treatment. NGS enables the simultaneous sequencing of millions to billions of DNA fragments in a single run. NGS can generate vast amounts of sequencing data in a relatively short period of time offering high accuracy and reproducibility with low error rates. NGS can also detect structural variants such as insertions and deletions. The upfront costs associated with NGS may be prohibitive for some researchers. NGS technologies generate short sequencing reads with high error rates ranging from 50 to 500 base pairs in length. They often exhibit uneven coverage across the genome with certain regions being over presented or under presented in sequencing data. | 3, 4 |
| Microarray |
This assists in understanding gene expression changes by binding of mRNA to sequences. The chip consisting of known DNA sequences is allowed to hybridise with complementary RNA sequences followed by detection using dyes to establish a comparative study on expression levels of a protein in more than one samples. |
5 |
| ELISA | Enzyme-linked immunosorbent assay (ELISA) is a highly sensitive technique used to study biomarker interactions in biological samples. In ELISA, biomarkers are immobilised onto a solid support, such as a microplate, and then exposed to specific antibodies conjugated with enzymes. These antibodies bind to the target biomarkers with high affinity and specificity, forming antigen–antibody complexes. After washing away unbound components, a substrate solution containing an enzyme substrate is added. If the target biomarker is present, the enzyme catalyses a colorimetric or fluorescent reaction, producing a signal directly proportional to the biomarker concentration. By measuring the intensity of the signal, researchers can quantify the amount of biomarker present in the sample. ELISA's versatility allows for the detection and quantification of various biomarkers, including proteins, peptides, hormones and antibodies, making it a valuable tool in biomedical research, clinical diagnostics and drug development. Its high sensitivity, specificity and scalability make ELISA an indispensable technique for studying biomarker interactions and elucidating their roles in health and disease. | 6, 7 |
| Western blotting | It helps in detection of specific proteins in a sample. It is based on binding of antibodies on the sample after electrophoresis, followed by visualisation of the proteins. The electrophoresed samples are transferred to a suitable membrane followed by blocking and addition and incubation of specific primary antibodies against the suspected protein. The secondary antibody against the primary antibody, either fluorescently labelled or associated with an enzyme, is added and incubated. The enzyme converts its substrate to a coloured compound whose intensity is measured and the protein is visualised. For fluorescent labelled secondary antibodies, the fluorescence is measured. | 8 |
| Cross-linking electrophoresis | Cross-linking electrophoresis is a technique utilised to investigate protein–protein interactions and complex formation among biomarkers. In this method, proteins are cross-linked with a chemical agent, forming covalent bonds between interacting molecules before subjecting them to electrophoresis. By applying an electric field, cross-linked protein complexes migrate through a gel matrix based on their size and charge. Subsequent analysis reveals the presence and nature of protein interactions based on the migration patterns of the complexes. This technique is particularly valuable in elucidating the interactions of biomarkers in biological systems, such as protein–protein interactions in signalling pathways or protein complexes involved in disease processes. By identifying and characterising these interactions, cross-linking electrophoresis provides insights into the molecular mechanisms underlying physiological and pathological conditions. Additionally, it enables the study of protein complexes' composition, stoichiometry and dynamics, aiding in the development of targeted therapeutics and diagnostic tools for various diseases. Overall, cross-linking electrophoresis serves as a powerful tool for understanding the intricate network of biomolecular interactions in living organisms. | 9, 10 |
| Mass spectrometry | It measures mass by charge ratio of one or more molecules present in a sample to determine molecular weight. It can be of two types—matrix-assisted laser desorption and ionisation (MALDI) and electron spray ionisation (ESI). Molecular weight is an important parameter of analysing different biomolecules and also help us to designate its functions based on molecular weight, such as, their ability to diffuse or get co-transported in the plasma membrane, their ability to react with other proteins. Molecular weight is also an important descriptor of structure and constituents. Both the mass spectrometry techniques use filters such as time of flight to screen and segregate a compound or a group having a specified mass from compounds, which are either lighter or heavier. The preparation of the samples requires careful implementation of standardised protocol to avoid error or ambiguity. | 11 |
| Karyotyping |
It involves staining and visualisation of chromosomes under a microscope to determine abnormalities and aneuploidies, which may lead to cancer at some point of time. These chromosomal abnormalities can be duplications, deletions or other aberrations, which change the transcriptomic and proteomic profiles of the cell. |
12 |
| Bioinformatics and computational biology | Here, various software and databases are used to analyse large datasets from genomic, proteomic and metabolomics studies to detect any qualitative change in the biomarkers. Different tools and platforms significantly reduce the spectrum of analysable data using different methods and strategies, which make evaluation of samples more rapid and accurate. | 13 |
| Nuclear magnetic resonance spectroscopy |
It applies magnetic field and radio frequency pulses to characterise resonant frequency of atomic nuclei according to its chemical or environmental surroundings. This frequency can be used to analyse metabolic changes and lipid profiles. This technique is also used for determination of three-dimensional conformation of a molecule, which helps us in gathering insight regarding the chemical formula and spatial arrangement of the constituents. |
14 |
| Confocal microscopy | Confocal microscopy is a powerful imaging technique used to study biomarker interactions with high spatial resolution and specificity. In confocal microscopy, a focused laser beam scans across the sample, illuminating a single plane at a time. Fluorescently labelled biomarkers emit light in response to the laser excitation, which is detected by a photo detector. By controlling the focal plane and excluding out-of-focus light using a pinhole aperture, confocal microscopy generates high-resolution, optically sectioned images of biomarker distribution and interactions within biological samples. This technique allows researchers to visualise biomarker localisation, co-localisation and dynamic interactions in live or fixed cells and tissues. Confocal microscopy is particularly valuable for studying biomarker interactions within subcellular compartments, such as organelles or protein complexes. By providing detailed insights into spatial relationships and dynamics of biomarker interactions, confocal microscopy contributes to our understanding of cellular processes, signalling pathways and disease mechanisms. Additionally, advancements in confocal microscopy, such as fluorescence resonance energy transfer (FRET) and fluorescence lifetime imaging microscopy (FLIM), enable quantitative analysis of biomarker interactions in real time, further enhancing its utility in biomedical research. | 15 |
| Magnetic resonance imaging (MRI) | MRI is a powerful non-invasive medical imaging technique that uses strong magnetic field, radio waves and a computer to generate detailed images of the body's internal structures. It is widely used in clinical practice for diagnosis, staging and monitoring of various medical conditions. MRI is also valuable for guiding minimally invasive procedures such as biopsies, surgeries and radiation therapy. MRI provides exceptional contrast resolution for soft tissues making it ideal for visualising organs, muscles and nerves within the body. It is a highly versatile procedure, which can be used to image virtually any part of the body. MRI offers multiple imaging sequences and techniques that can provide complementary information about tumour biology. MRI tends to be more expensive than other imaging modalities such as X-ray or ultrasound. They are not widely available in non-developed areas such as rural areas. MRI images may be susceptible to distortion and degradation due to patient motion, metallic objects which may be attracted due to strong magnetic field inside MRI machines. | 16, 17 |
| Computed tomography | Computed tomography (CT), also known as computed axial tomography (CAT) scanning, is a medical imaging technique used to create detailed images of the body's internal structures. CT imaging is valuable for detecting tumours, assessing their size, location and spread (staging), and monitoring treatment response. CT scans provide detailed images with high spatial resolution and excellent tissue contrast, allowing for the accurate visualisation of anatomical structures and pathological abnormalities. CT imaging is non-invasive and well tolerated by patients. CT scans can be reconstructed into three-dimensional (3D) images, allowing for better visualisation of complex anatomical structures, surgical planning and educational purposes. Although the radiation doses used in modern CT scanners are generally considered to be low, repeated exposure to ionising radiation over time can increase the risk of cancer, particularly in sensitive populations such as children and young adults. CT scans are generally avoided during pregnancy, especially during the first trimester, due to the potential risks of radiation exposure to the developing foetus. | 18-20 |
| Microfluidics | It is an emerging high throughput technology based on analysis of fluids by manipulating them using small channels, which are generally micrometres in size. In a microfluidic system, fluids can be mixed, separated, transported or otherwise processed. The design of the systems that allows processing of low volumes of fluids makes automation and multiplexing achievable. | 21 |
| Fluorescence in situ hybridisation (FISH) | It is one of the most popular technique used for discovering specific DNA sequences, recognising oncogenes or genetic aberrations which are responsible for cancer, genetic mapping and diagnosis of various genetic diseases. It allows us to detect the locations of hybridisation between a fluorescent probe and denatured DNA sample by microscopic observation. | 22 |
- These techniques have a wide range of applications depending on the aim of investigation and type of sample, which is analysed. In many cases, a combination of techniques are used for accurate comprehension of the levels of biomarkers in the sample. These techniques have their own advantages and limitations in evaluating the different biomarkers, and therefore, exploration and analysis of the biomarkers are performed using more than one technique consecutively. All these techniques provide results, which can be conveniently interpreted and valuable information regarding the status of cancer in an individual, which helps in understanding the present stage of cancer, predicting the future of cancer in the patient and designing possible therapies for cure. These techniques are highly sensitive, have magnificent selectivity, accuracy and precision, provide real-time analysis, are high throughput and have a wide range and good reproducibility. However, interference, problems in sample preparation, lack of operational expertise and cost are some of the limitations of these techniques, which should be significantly minimised.
The clinical applications of cancer biomarkers aim for precision medicine, enhancing prevention, screening and treatment strategies. Biomarkers are utilised for risk assessment, early detection, accurate diagnosis, patient prognosis, predicting therapy response and monitoring cancer. They encompass DNA repair assays, genotyping for germline variants and LB-based tests. Additionally, biomarkers aid in treatment decision-making, helping identify probable responders or non-responders and guiding therapy adjustments. Monitoring biomarkers, such as ctDNA, offer potential for real-time disease tracking during and after treatment. Discovery involves identifying and prioritising potential biomarkers using advanced technologies like NGS and gene expression arrays, while ensuring proper study design and data analysis. Assay development focuses on creating a protocol for biomarker detection and validating its accuracy. Clinical validation assesses the biomarker's ability to predict clinical outcomes, requiring external validation and statistical analysis. Clinical utility examines whether using the biomarker improves health outcomes, typically through prospective trials. Clinical implementation involves regulatory approval, commercialisation and integration into healthcare systems, with challenges including standardisation and validation. Cancer biomarkers, traditionally from tumour tissue, now include DNA, RNA and protein-based markers from body fluids due to recent research. NGS enables comprehensive genetic analysis, emphasising the benefits of studying both germline and somatic mutations. However, challenges include test optimisation and the low concentration of biomarkers in body fluids. To address this, sensitive detection technologies like nanoparticles with sensor technology are being developed. Regulatory approval requires rigorous validation and assessment of clinical utility before clinical implementation.
Cancer is a global health concern, with millions of new cases and deaths annually. Detecting cancer early is crucial for effective treatment, but current methods face challenges. LB, including circulating tumour DNA (ctDNA), shows promise for non-invasive detection.25 Despite its potential, challenges remain, such as the need for highly sensitive assays. DNA methylation, an early event in cancer, offers advantages as a biomarker, including tissue specificity and consistency across genomic regions. Recent advances in ctDNA methylation analysis hold promise for cancer diagnosis and prognosis. Cell-free DNA (cfDNA), comprising nucleic acids released into the bloodstream, can reflect genetic and epigenetic information from tumour cells. The concentration of ctDNA varies and correlates with tumour stage. Understanding these dynamics is essential for developing sensitive detection methods.25 ctDNA methylation analysis holds promise in oncology, yet faces hurdles in sensitivity, specificity and standardisation.25 Challenges include limited ctDNA availability, variability in methods and biological influences. Analyte selection and large-scale multi-centre studies are crucial for clinical validity. Future improvements may involve integrating various biomarkers and leveraging machine learning for analysis.
According to Nikanjam et al., liquid biopsies, including ctDNA and CTCs, are crucial for predicting patient outcomes post-surgery. ctDNA levels correlate with worse survival, while identical mutations in tissue and ctDNA indicate shorter survival. Additionally, the presence of ctDNA predicts recurrence and poor survival in various cancers, aiding in treatment decisions and monitoring.26 LB also serves as a valuable tool for assessing hard-to-biopsy patients and detecting early-stage cancers, offering potential for timely intervention and improved outcomes.
Chimeric antigen receptor (CAR-T)-cell therapy, increasingly used for haematologic malignancies, shows remarkable responses. Predicting response and monitoring progression beyond imaging and bone marrow biopsy is crucial for better outcomes. A pilot study using blood-derived genomic instability number (GIN) analysis found that patients with aggressive B-cell lymphomas who maintained complete response had GIN levels below 170. Increasing GIN levels preceded imaging diagnosis of relapse or progression in patients with disease recurrence, suggesting cfDNA could predict early relapse and monitor response post CAR-T infusion.26 Further research is needed. Liquid biopsies, a cutting-edge technology with vast clinical applications, have revolutionised tumour molecular profiling and precision medicine. Tumours release ctDNA/cfDNA and CTCs into the bloodstream, which are analysed through various methods like ddPCR and whole-genome sequencing. These biomarkers offer insights into immune checkpoint blockade responses, treatment predictions, tumour heterogeneity and pharmacological alterations. While easier to isolate, ctDNA/cfDNA may be limited by small quantities and CHIP interference, unlike CTCs, which offer multi-dimensional evaluation and functional assessment. Liquid biopsies, being less invasive and more cost-effective than tissue biopsies, provide real-time molecular information critical for patients with hard-to-biopsy tumours.26 Despite their potential, challenges like standardisation and regulatory issues persist. The future of liquid biopsies lies in exploring alternate fluids and additional analytes for early cancer detection, promising to transform oncology management.
3 CLINICAL MANIFESTATIONS DUE TO CHANGES IN BIOMARKERS
-
Prostate-specific antigen (PSA): PSA, also known as gamma-seminoprotein or kallikrein-3 (KLK3), is a glycoprotein enzyme coded by the KLK3 gene. This antigen is secreted in men's prostate epithelial cells and women's paraurethral glands. It helps in the liquefaction of semen helping in sperm motility as well as dissolving cervical mucus, both of which assist in sperm motility. Usually, PSA is present at low levels. However, increased amounts may suggest prostate cancer or benign prostatic hyperplasia. Clinical manifestations of increased levels include urinary difficulties, haematuria (blood in urine) and pelvic discomfort.28
-
Carcinoembryonic antigen (CEA): Produced in the GI tissue mostly during foetal development, CEAs in a broad sense include a series of highly related glycoproteins assisting in cellular adhesion. Consequently, healthy adults have roughly 2–4 ng/mL CEA in their blood and LB samples. High levels of CEAs in LB samples can be an indication of CRC. Elevation is observed less for organ confined tumours. The main reason for this is that these antigens possess sialofucosylated glycoforms that act as functional L-selectin and E-selectin ligands. Clinical symptoms include weight loss, abdominal pain, changes in bowel habits and rectal bleeding.28
-
Alpha fetoprotein (AFP): AFP is another protein produced during the foetal stage as well. It is encoded by the AFP gene and has no known function in adults’ despite being a carrier protein during foetal development. High AFP levels in adipose tissue can be indicative of liver cancer (hepatocellular carcinoma [HCC]) and germ cell tumours as well as other birth defects. Clinical symptoms include jaundice, abdominal pain, weight loss and fatigue.28
-
CA-125: CA-125 or Cancer Antigen-125 is encoded by the MUC-16 gene. It is a component of the ocular surface, respiratory tract and female genital epithelia where it helps create a hydrophilic lubricating barrier against foreign agents thanks to its glycosylated nature. Elevated levels of this antigen if detected during LB can be an indicator of ovarian cancer since it advances tumour proliferation and metastasis via expression of cytoplasmic tail and ability to participate in intercellular interactions.28 Additionally, it can also reduce sensitivity of cancer cells to drug therapy. Common manifestations include abdominal bloating, pelvic pain, early satiety (feeling full quickly) and changes in bowel habits.28
-
CA 19-9: CA 19-9 is an antigen hailing from the family of tumour markers. Increased CA 19-9 levels are associated with pancreatic cancer. Clinical manifestations include jaundice, abdominal pain, weight loss and new-onset diabetes.28
-
Vascular endothelial growth factor (VEGF): VEGF is a signal protein, derived from platelets, that is responsible for controlling and stimulating blood vessels. Increased VEGF levels in liquid biopsies suggest enhanced angiogenesis, which is crucial for tumour growth and metastasis.28 Clinical symptoms depend on the cancer type but may include increased tumour size and metastatic spread, leading to organ-specific symptoms.
-
Oestrogen receptor (ER) and progesterone receptor (PR): As their names suggest ER and PR are responsible for binding with oestrogen and progesterone enzymes of the females. They are most common in the breast cells, both normal and tumour ones, since these hormones help in their growth and development. Changes in their expression in adipose tissue due to structural mutation are associated with breast cancer (BC).28 Clinical manifestations include changes in breast shape, breast lumps, nipple discharge and skin changes over the breast.
-
Ki-67: Ki-67 is a protein found only in dividing cells. An increased expression of it in the adipose tissue indicates high cellular proliferation rates, often seen in aggressive cancers. Clinical manifestations depend on the type and location of the cancer but generally include rapid tumour growth and potential metastasis.28
4 INSTANCES OF APPLICATIONS OF LIQUID BIOPSY
4.1 Applications of liquid biopsy in cancer and personalised medicine
-
Immunomagnetic methods: Systems like CellSearch capture CTCs based on specific antigens, providing high purity but may miss cells without these markers.
-
Microfluidic systems: Devices like ClearCell FX sort CTCs and cfDNA by physical properties rather than antigens, offering high throughput but with potentially lower specificity.
-
Filter-based techniques: Methods such as ScreenCell use size-based filters to quickly capture larger DNA fragments and CTCs, but may lack specificity for smaller or atypical cells.
-
Advanced imaging and dielectrophoresis: Techniques like DEPArray use imaging and electric fields for precise isolation, offering high detail but at higher costs and complexity.
-
Density gradient centrifugation: Methods like OncoQuick use density differences to separate cfDNA and CTCs, balancing yield and purity, though they may require longer processing.
-
Advanced nucleic acid analysis: Technologies like digital droplet PCR and NGS provide detailed mutation detection and genomic profiles, essential for monitoring and treatment planning.
Blood-based liquid biopsies use blood samples to detect biomarkers like ctDNA, CTCs and miRNAs. These biomarkers are particularly useful for monitoring cancers such as non-small-cell lung cancer (NSCLC) and metastatic BC, and in prenatal diagnostics for early genetic disorder detection.29 Urinary liquid biopsies analyse urine to monitor urological cancers by detecting cfDNA, miRNAs, proteins and peptides. New techniques like Attenuated total reflectance-Fourier transform infrared spectroscopy are improving the accuracy of these tests.29 Salivary liquid biopsies offer a convenient method for cancer detection, particularly for gastric and oral cancers, using ScfDNA. This approach is advancing with techniques that differentiate cancerous from non-cancerous states through DNA fragment analysis. Cerebrospinal fluid (CSF) is used for brain cancer diagnostics, allowing for sensitive detection of tumour-specific mutations, though challenges like sample collection and variability remain.29 Breast milk is emerging as a medium for LB in postpartum BC detection, leveraging ctDNA to identify tumours early.29 Pleural effusion analysis is improving cancer diagnostics by differentiating malignant from benign conditions and offering insights into tumour biology through exosomal miRNAs and other biomarkers.29 Overall, LB techniques are enhancing cancer detection, monitoring and treatment personalisation, representing a significant advance in precision medicine and proactive healthcare.
4.2 Liquid biopsy in clinical management of cancers in brain, breast and gut
According to recent research, paediatric low-grade gliomas (pLGG) are the most common brain tumours in children.30 They are initially treated with surgery, and about half of patients need further therapy. Historically, this has been chemotherapy or, less often, radiotherapy. While the 10-year survival rate is generally high, tumours in the thalamus or brainstem have lower survival rates, and radiotherapy can increase risks.30 New molecular diagnostics have revealed common mutations, such as V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF) V600E, which are linked to poorer responses to standard chemotherapy. However, mitogen activated protein kinase-inhibitor trametinib, especially when combined with BRAF-inhibitor dabrafenib, shows better results.30 This combination therapy, recently Food & Drug Administration (FDA)-approved, is more effective than chemotherapy. Molecular diagnosis is crucial for treatment, and when surgery is not possible, liquid biopsies offer a less invasive alternative. LB analysis confirms the presence of tumour DNA in CSF, guiding effective treatment decisions. Reports demonstrated that in the last decade, cancer immunotherapy has become a significant treatment option alongside traditional methods like chemotherapy, radiotherapy and surgery. Immune checkpoint inhibitors (ICIs), which target proteins like PD-L1, PD-1 and CTLA-4, help to restore T-cell function and boost the body's ability to fight tumours.31 Drugs such as atezolizumab (anti-PD-L1), pembrolizumab (anti-PD-1) and ipilimumab (anti-CTLA-4) have been approved by the FDA and European Medicines Agency for various cancers.31 BC is the most common and deadliest cancer in women. Historically, BC has been less responsive to immunotherapy compared to other cancers. However, recent advances have shown promising results with ICIs, particularly in triple-negative breast cancer (TNBC). Trials like Impassion 130 and Keynote 355 highlight the benefits of ICIs in BC treatment.31 Currently, the effectiveness of ICIs is often assessed through PD-L1 expression levels using immunohistochemistry (IHC), but this method has limitations due to variability in test results, different assay platforms and tumour heterogeneity. Therefore, LB, which analyses components in blood such as CTCs and DNA, is emerging as a promising alternative for guiding immunotherapy in BC. LB offers a less invasive and faster approach compared to traditional biopsies. Ongoing research aims to refine LB methods to better predict treatment responses and optimise patient care. CTCs in the blood can be detected using biological and physical methods. The CellSearch™ system, the only FDA-approved method for detecting CTCs in metastatic BC, isolates these cells with magnetic beads and distinguishes them from other cells using specific stains.31 Studies show that CTCs are linked to changes in the tumour immune environment, such as increased regulatory T cells and reduced dendritic cells, which affect immune response. New technologies now allow detailed analysis of CTCs, revealing markers that can predict responses to immunotherapy and helping monitor treatment effectiveness. ctDNA, which comes from tumour cells, is found in higher concentrations in the blood compared to normal cfDNA. It is a promising non-invasive tool for early cancer detection and monitoring treatment outcomes. Studies have shown that ctDNA levels can indicate the effectiveness of therapies like pembrolizumab in BC and other cancers.31 Decreasing ctDNA levels after treatment are linked to better outcomes, while increasing levels suggest disease progression. ctDNA can also provide insights into how tumours resist treatment.31 New techniques are improving the detection of low levels of ctDNA, offering valuable information for managing cancer. EVs are crucial for cell communication and affect both cancer and normal cells. Tumour-derived EVs often suppress the immune system, helping tumours avoid detection by down regulating important immune signals and causing various immune cells to become less effective. However, EVs can also activate the immune system by stimulating certain immune cells, leading to better tumour clearance in some cases. EVs are being explored as biomarkers to predict treatment responses in cancer patients.31 Despite the potential, standardised methods for isolating EVs from blood are needed for effective clinical use. PD-L1, also known as CD247 or B7-H1, is a protein found on immune and tumour cells. It interacts with PD-1 on T cells to inhibit their activation, which reduces the immune system's ability to fight cancer.31 Monoclonal antibodies that block the PD-1/PD-L1 interaction have been effective for patients with high PD-L1 levels in tumours. However, some patients with low PD-L1 levels also benefit from these treatments, likely due to the limitations of static tests like IHC. The expression of PD-L1 in both cancer cells and immune cells complicates its measurement. Variability in detection methods and differences between primary and metastatic tumours further challenge tissue-based assessments. Blood-based tests for PD-L1, such as analysing CTCs and exosomes, may offer more dynamic insights. Studies show that PD-L1 on CTCs can vary widely and reflect treatment responses and disease progression.31 Tumour mutational burden (TMB) is no longer a standard biomarker for treatment selection, but it may still indicate T-cell activation and predict response to ICIs. Traditionally, TMB was assessed using tumour biopsies, but alternative methods like liquid biopsies (ctDNA and CTCs) are emerging, especially when tissue samples are limited.31 Studies show that blood-based TMB (bTMB) correlates with tissue-based TMB and can predict treatment efficacy. For patients with metastatic non-NSCLC, bTMB is a practical alternative when biopsies are challenging.31 However, differences between tissue and blood TMB need further investigation to establish standardised assessment methods.
Mismatch repair (MMR) is crucial for DNA accuracy. When MMR is deficient, microsatellite instability (MSI) can occur, which is common in various cancers. In CRC, tumours with dMMR/MSI often respond well to PD-1/PD-L1 inhibitors like pembrolizumab. While MSI/dMMR is rare in BC, it is seen more in high-grade, low PR tumours. These cases may benefit from ICIs, such as nivolumab or pembrolizumab.31 MSI can also be detected through ctDNA, with high MSI levels correlating with better responses to these treatments.
The effectiveness of ICIs depends on T cells recognising neoantigens, which are displayed by major histocompatibility complex (MHC) molecules. T-cell receptors (TCRs) identify these neoantigens as foreign, prompting an immune response. Analysing the TCR repertoire, specifically the unique CD3 region, can predict how well ICIs will work. Studies have shown that changes in the TCR repertoire in blood, such as increased diversity after chemotherapy, are linked to better treatment outcomes.31 TCR sequencing from blood can thus be used to gauge the success of immunotherapy in BC patients.
CTCs and cfDNA are promising biomarkers for cancer management. Beyond detecting mutations in cfDNA, new methods are being developed. One approach, ‘fragmentomics’, analyses cfDNA fragmentation patterns to differentiate between cancer patients and healthy individuals.32 Another technique, methylation sequencing, examines methylation patterns to detect cancer presence and type.33 Chromatin state analysis and nucleosome foot printing study nucleosome positioning and transcription factor binding to identify cancer subtypes.34 However, detecting tumour-derived cfDNA is challenging, particularly in tumours with low mutational burden like BC. These new methods offer broader genomic insights compared to traditional mutation panels and may enhance cancer treatment monitoring.
LB shows potential for better managing BC and improving survival rates. Advances in molecular analysis have expanded its use for diagnosis and treatment prediction. However, challenges remain. CTCs are rare and require sensitive equipment for detection, while ctDNA is often present in very low amounts and can be difficult to analyse. New technologies and machine learning might improve detection. Combining ctDNA analysis with other biomarkers and assessing additional features could enhance accuracy. Standardisation of LB practices, through guidelines and quality control, is needed for reliable and consistent results across labs.
Immunotherapy is a potent treatment for BC, but responses vary among patients. Identifying specific patient groups and using biomarkers for personalised treatment is crucial. Liquid biopsies are useful for evaluating immune-related biomarkers in BC.35 Research is ongoing for PD-L1 on CTCs and exosomes, while cfDNA and ctDNA are analysed with advanced technologies. The role of TMB in immunotherapy needs further clinical validation. Evidence shows genomic markers like MSI and TCR in blood correlate with the effectiveness of ICIs.35 Liquid biopsies offer benefits such as repeated sampling, simplicity and minimal invasiveness, aiding in treatment monitoring and adjustment. Although not yet a formal guideline, LB's non-invasive nature makes it a promising tool for optimising immunotherapy in BC.
GI cancers, including colorectal, stomach, oesophageal, liver and pancreatic cancers, are common and vary in global incidence and mortality. They account for about one-third of cancer cases and deaths worldwide, with expected increases in the future. These cancers significantly impact quality of life, making improved care crucial. CRC is one of the most common, but its mortality rates are decreasing due to better treatments and early detection. Stomach cancer, despite a decreasing incidence in many areas, still has high mortality due to late diagnosis. Oesophageal cancer shows varied incidence and high mortality due to late detection, while liver cancer has high mortality due to late-stage diagnosis and factors like viral infections. Pancreatic cancer has the lowest survival rates because it is often diagnosed late. Early detection methods, such as liquid biopsies, are critical for improving outcomes. Liquid biopsies analyse biomarkers from bodily fluids like blood, offering a non-invasive way to detect cancer-related changes and monitor disease progression. They are especially useful for tracking treatment responses and capturing tumour diversity.36 CTCs from primary or metastatic tumours can indicate the presence of cancer such as HCC and pancreatic ductal adenocarcinoma (PDAC), and help in early diagnosis, although current methods to detect CTCs are not yet widely implemented due to their rarity and technical challenges.36 CTC evaluations are useful for monitoring therapy and predicting relapse or progression across various cancers.
Platelets are key blood cells crucial for wound healing and homeostasis, originating from megakaryocytes. Tumour-educated platelets (TEPs) are those that interact with tumour cells and collect tumour-associated biomolecules and vesicles. This interaction suggests TEPs play a significant role in cancer progression and prognosis. Research shows that TEPs can carry RNA profiles and other biomarkers from tumours, making them valuable for cancer diagnostics.36 They have been observed in various cancers, including lung, breast, liver and brain cancers, showing their potential in early diagnosis and treatment monitoring. Though the role of TEPs in cancers like CRC is less explored, they hold promise in distinguishing between cancer and non-cancerous conditions, and might be useful alongside other biomarkers.36
Circulating cfDNA consists of DNA fragments circulating in blood, primarily from normal cells, but also from tumours. Elevated cfDNA levels in cancer patients make it a useful marker for tumour detection. Tumour-specific mutations found in cfDNA, known as ctDNA, are important for identifying cancer and tracking treatment responses. ctDNA levels and mutation profiles are used to diagnose and monitor various cancers, including CRC and HCC. Studies suggest that cfDNA and ctDNA can detect cancer earlier than some traditional methods and may improve prognosis and treatment strategies.36
ctDNA derived from tumour cells is a promising tool for cancer detection and monitoring. It can reveal tumour mutations and help in assessing treatment efficacy. Despite challenges in detecting low quantities of ctDNA, its use in early cancer detection and disease monitoring is growing.36 ctDNA's potential in predicting cancer and guiding treatment decisions is supported by its ability to reflect tumour dynamics and heterogeneity, especially in advanced stages. Studies suggest ctDNA can significantly improve early diagnosis and personalised therapy in cancers, including GI cancers.36
Exosomal miRNAs are small RNA molecules that regulate tumour growth. They are involved in cancer progression and can be used for diagnosis and prognosis. Certain exosomal miRNAs are linked to CRC and can indicate tumour presence, stage and treatment response. For instance, high levels of miR-21 and miR-1246 are associated with CRC. Panels of these miRNAs offer better diagnostic and prognostic value than single markers.36 LncRNAs are RNA molecules that do not encode proteins but can affect tumour behaviour. Exosomal lncRNAs, such as HOTTIP and FRLnc1, are being studied for their potential as biomarkers in GI cancers.36 They can provide insights into cancer progression and treatment response. Circular RNAs (circRNAs) are stable RNA molecules with closed loops. They play roles in regulating gene expression and are stable in exosomes. CircRNAs like has_circ_0004771 and circ-FBXW7 are being explored for their potential in cancer detection and chemoresistance.36 Proteins in exosomes can also serve as cancer biomarkers. Elevated levels of proteins like MIF and GPC-1 are associated with pancreatic cancer, while changes in proteins like CPNE3 and QSOX1 are linked to CRC prognosis.36 Enhanced isolation methods and multi-analyte panels could improve accuracy and reliability of LB, helping it become a valuable tool for early cancer detection and monitoring.
5 CONCLUSION
The wide range of techniques that can be used to analyse the levels of biomarkers associated with cancer in a patient's body makes LB a promising technique for cancer therapy.37 Understanding the mechanisms by which changes in biomarker levels occur in a patient, the pathways which get affected and the consequent manifestations are very important for proper treatment of cancer. Through various sophisticated and advanced technologies, LB ensures that scientists and medical experts get comprehensive insight on different types of cancer tormenting humans for decades.38 LB is transforming cancer diagnosis and management by analysing various bodily fluids. Unlike traditional biopsies that require surgery, LB only needs a blood sample, making it less invasive and allowing for frequent monitoring. This method can be used with other fluids like urine, saliva and CSF, each providing unique diagnostic information for different cancers.29 For instance, urine biomarkers can indicate urological cancers such as bladder and prostate cancer. LB also tracks the genetic changes in tumours over time, helping assess treatment effectiveness and adjust therapies promptly. It captures a broad range of genetic material from tumours, offering a comprehensive view that traditional biopsies might miss. Additionally, microRNAs and EVs provide further insights. MicroRNAs reflect the tumour's biological state, while EVs carry tumour-specific proteins and genetic material. These components enhance diagnostics, especially when other tumour markers are hard to isolate. Overall, LB improves cancer detection and treatment by providing detailed and continuous insights into tumour dynamics, leading to better patient outcomes and personalised care.29 Liquid biopsies are promising for cancer detection and personalised treatment but face several challenges. Key issues include balancing sensitivity (detecting all disease instances) and specificity (avoiding false positives). This balance is hard to achieve due to the low levels of ctDNA and the presence of other genetic materials. Tumour diversity adds complexity, as liquid biopsies may miss some genetic variations of tumours. Technical difficulties arise from the low concentration of ctDNA and CTCs in biofluids like blood, urine and saliva. Additionally, isolating miRNAs and EVs, which are small and variable, requires precise methods. Contamination risks, tumour dynamics and economic and regulatory concerns also impact LB effectiveness. Addressing these issues is essential for fully utilising liquid biopsies in cancer care. Liquid biopsies have been met with both hope and scepticism. One myth is that they cannot detect a wide range of genetic markers compared to traditional biopsies. In reality, they can capture a broad genetic profile and provide insights into tumour evolution.29 Another myth is that miRNAs and EVs are inferior biomarkers. Many studies show that they offer valuable diagnostic and prognostic information. Liquid biopsies complement rather than replace traditional methods, allowing for real-time monitoring of genetic changes, however, their effectiveness varies by cancer type and stage.29 LB technology is advancing rapidly. Innovations like NGS improve genetic precision, while machine learning and artificial intelligence (AI) enhance mutation analysis. Microfluidic technologies are speeding up sample processing, expanding the scope of liquid biopsies to early cancer detection and personalised treatments. New tests can detect multiple cancers from a single blood sample and are being explored for non-cancerous conditions. Advances in miRNAs and EVs could make them key to personalised medicine. Liquid biopsies might also improve pathogen detection in infectious diseases. Ongoing research and technological progress will continue to refine these methods, aiming to revolutionise diagnostics and patient care.
AUTHOR CONTRIBUTIONS
Subham Sarkar—conceptualisation, preparation of the final draft, illustration and formatting; Samraggi Chakraborty—writing of the original draft; Soubhagya Ghosh—writing of the original draft; Ekanansha Roy Chowdhury—writing of the original draft; Jenifer Rajak—illustrations; Bikram Dhara, Arup Kumar Mitra and Ajoy Kumer—Conceptualisation, Editing the original manuscript, overall supervision, Project administration.
ACKNOWLEDGEMENTS
The authors have nothing to report.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable for this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created in this study.




