Engineered cell versus modified exosomes in cancer therapy
Abstract
Cancer therapeutic development is the most challenging domain in cancer. Cell-based cancer therapeutics come up with promising effectiveness. This approach was also cell-modified for better targeting efficiency development. Cell engineering-based cancer therapeutic is a cutting-edge method in cancer therapy. Due to complications of this process, cost and post-treatment side effects, this phenomenon came into the question mark. In this scenario, extracellular vesicle (EVs) research introduces a cell-free cancer therapeutic approach. In the therapeutic aspect most used EVs, come from stem cells, plants, and engineered cells. Among several EVs populations, Exosomes are the most used worldwide cell-free therapeutic tool for ageing cancer. The most interesting facts about exosomes are the biocompatible, non-immunoreactive, cross-biological barrier, and non-toxic (depending on the parental cell's nature). In this article, we are exploring modified exosomes (biological or chemical) that create a remarkable outcome in cancer therapeutic development compared to engineered cell-based therapeutics. Hope, in the future, modified exosomes become an effective, affordable, and specific cancer-targeting precision medicine.
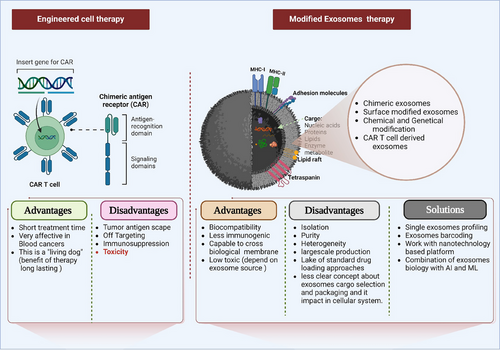
Cancer-associated healthcare burden is the most complicated problem for human civilization.1 Traditional cancer therapeutic approaches such as surgery (it sheds the cancer cells into the circulation system and promotes cancer metastasis), radiotherapy (leads to enrichment of reactive oxygen species which plays a role in mutation in cells and triggers cancer stem cells development), chemotherapy (chemotherapy-exposed tumour cells release exosomes related to premetastatic signalling, and chemotherapy suppresses T cell activity against cancer), and immunotherapy(immune check point-based immunotherapy, but, this approach is very expensive) are taking a back seat in this cancer battle due to its limitations. Thus, scientists are searching for new approaches to treat cancer which can replace the already existing therapies. Chimeric antigen receptor (CAR) -T cell is an impressive cell engineering (Isolation of T cells from the blood of the patient—genetically engineering T-cell via CAR and culturing the cells and —reinjecting to the patient) approach for cancer treatment. However, this is not a fool proof approach as it is associated with a number of side effects such as low blood pressure, fever, allergic reaction, abnormality in the presence of minerals in the blood, impact on the nervous system (confusion and speaking trouble), toxicity, antigen escaping and immunome suppression.2 Overcoming the hurdles, extracellular vesicles (EVs) have gained prominence by addressing all of these limitations and providing a new orientation. EVs are released by all living cells and the major subpopulations of EVs are microvesicles, apoptotic bodies and exosomes. In the current decade, exosomes has been identified as one of the best alternatives for cancer theranostic. Tumor-derived exosomes (TEXs) play a significant role in cancer development and progression. TEXs reprogram immune cells (induce M2 polarization, and the reduce anticancer cytotoxic effect of cytotoxic T cells, dendritic cells, and NK cells) and promotes cancer.3 Scientific evidences mention that exosomes integrins regulate organ-specific metastasis.4 Exosomes-based cancer therapeutic development based on stem cell exosomes (it has a dual nature, both tumor promoting and suppressing action), plant exosomes (a non-toxic source and these exosomes contain several phytochemicals, but the working principle of these are still not clear), immune cell-derived exosomes (dendritic cell-derived exosomes are the most effective one but it activates only natural killer cell-based immune responses), tumor cell-derived exosomes (this is not recommended due to its enrichment of high oncogenic cargos) and modified exosomes (chemically or genetically modified). 5 Engineered cells-derived exosomes (Figure 1) overcome toxicological limitations and reduce cancer development.6, 7 This approach too faces challenges in its isolation, heterogeneity, and toxicity.8 However, modified exosomes have a great clinical impact. In this process, exosomes are modified dynamically and these include surface modifications (target-specific ligands), and inner cargo loading (DNA, RNA, drugs, and proteins).9 Chimeric Exosomes is a cutting-edge approach for cancer therapy. In this method lab synthesised phospholipid bilayer membranes, drugs and a combination of antiphagocytic proteins CD14 (isolated from red blood cells) are developed into chimeric exosomes10. Based on scientific investigation it indicates that this is biocompatible in both in-vitro and in-vivo models.10 Modified exosomes has created a big hope in the cancer therapeutic domain. Though engineered cells derived exosomes is a promising approach, further studies are required to assess its toxicological safety and its use in cancer therapeutics 7. Moreover, exosomes biology has multiple technical limitations (Standard Isolationprotocols have to be optimized, drug loading standard approaches, requires more clinical trials, exosomes dose determination and route of administration needs to be determineds, storage methods, exosomes aggregation based off target phenomena) and therefore requires investigations to label it as an efficient approach for future cancer therapy. Single exosome-based profiling is also a smart approach for specific investigation which includes a combination of advanced nanotechnology, multiomics, artificial intelligence (AI) and machine learning (ML). In this process, nanotechnology solved exosomes isolation and characterization-related issues, multi-omic profiling of exosomes revealed molecular diversity and specific biomarkers and AI and ML support exosomes-based clinical data analysis. Another e approach has been developed, called exosome barcoding. This is a simplified version of single exosome profiling, and works based on four ways such as physical (electrochemical and optical based assays), chemical (based on different dyes and probes), Nano particle-based (e.g. quantum dot) and biological barcoding (DNA and antibody conjugation). Exosome barcoding is a user-friendly, sensitive and efficient way of exosome screening. In future, exosome biology and nanotechnology integration can create a new era for cancer precision medicine. Modified exosomes thus offer a promising platform for effective, efficient, and affordable cancer therapeutic development compared to engineered cell therapy.
ACKNOWLEDGEMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
There is no funding for this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.




