Targeting glutamate metabolism in chronic lung diseases
Abstract
Amino acids are necessary for all life forms, which play various roles. Disorder of amino acid metabolism is now considered an important driving mechanism in diverse pulmonary conditions, particularly chronic lung diseases like chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis and lung cancer. Glutamate actively participates in multiple vital biological processes, while the intricate glutamate metabolism, glutamate receptors and glutamate transporters assume crucial regulatory functions in the development of chronic lung diseases. This review aims to discuss the relationship between glutamate dysfunction and chronic lung diseases. By discussing the physiological and pathological function of glutamate, we probe the potential drug targets for chronic lung diseases in the glutamate pathway.
1 INTRODUCTION
The lungs are vital organs involved in gas exchange and are largely exposed to various disease risk factors such as air pollution, cigarette smoke and occupational contaminants. Hyperoxic environments, large surface areas and adequate blood supply may increase the risk of lung damage due to oxidative stress and other factors. Chronic lung diseases such as chronic obstructive pulmonary disease (COPD), asthma and idiopathic pulmonary fibrosis pose significant global health challenges and demand effective treatment solutions due to the substantial burden they impose.1 Despite the distinct pathophysiological mechanisms underlying each condition, they exhibit significant commonalities such as the existence of airway inflammation, airflow obstruction, airway remodelling and mucociliary dysfunction.2 Despite important advances in therapy for these lung diseases, there remain unmet clinical needs for targeted and highly effective treatment. Metabolomics, which focuses on small molecule metabolites such as amino acids (AAs), lipids, fatty acids and carbohydrates, holds great promise for identifying biomarkers for chronic lung diseases due to its high sensitivity, specificity and accuracy.3-6 Maintaining metabolic homeostasis is crucial for normal cellular structure and function, as any imbalance can lead to various cell injuries and even cell death, ultimately affecting the integrity of lung function and contributing to the development of lung diseases. Current research is striving to incorporate metabolomics as part of a comprehensive approach for disease diagnosis and prognosis.7
Proteins are formed from AAs, which act as fundamental units, and they have significant implications in cellular metabolism. Disorders related to the metabolism of AAs can potentially contribute to the advancement and onset of various ailments, including cancers, cardiovascular and renal diseases, as well as haematological and neurological disorders. The metabolisms of AAs are a complex process with tissue and organ-specific patterns. Depending on the functions and pathological conditions of tissue and organs, the AA can change their metabolism. In the past few years, the clinical application of targeted AA metabolism has attracted increasing interest in medical science. Considering the strong correlation of AA metabolism with inflammation and immunity, several studies have been initiated in the field of chronic lung disease research.8-12 Some AAs have been used as severity or prognosis markers. Dysfunction of these AAs can affect body homeostasis giving rise to diverse human diseases. Understanding the specific roles of these AAs in the pathogenesis of this disease may help to develop potential therapeutic approaches that target these molecules.
2 THE STRUCTURE AND FUNCTION OF GLUTAMATE
Among the free AAs, glutamate stands out as a highly prevalent compound, serving as a primary excitatory neurotransmitter within the central nervous system. Moreover, it plays a vital role in essential biochemical functions, encompassing oxidative stress, protein synthesis, energy metabolism and the metabolism of other AAs.13 The important characteristics of glutamic acid are as follows: (1) Its chemical structure is stable; (2) It is associated with the metabolism of numerous substances; (3) It is negatively charged.14 Glutamate is an essential component of proteins and can also be converted into other AAs, including glutamine, aspartic acid and alanine.15 Transformations between glutamate and other AAs are mainly manifested in the fact that most AAs can transfer their amino groups to α-ketoglutarate (α-KG) and polymerise to form glutamate, while in turn glutamate can form any of the corresponding AAs by supplying its amino group to a wide range of keto acids.13 Glutamate can be metabolically produced and cleared through interconversion with α-KG to participate in the metabolism and conversion processes of a wide range of substances. It is converted into α-KG by glutamate dehydrogenase (GLUD) and/or transaminase and then enters the tricarboxylic acid (TCA) cycle to provide carbon for oxaloacetic acid, acetyl-coenzyme A, citrate production and lipogenesis and nitrogen for purine, pyrimidine and DNA synthesis16, 17 (Figure 1). Glutamate is comprised a molecular structure that includes a carboxyl group (COOH), an amino group (NH2) and a distinct side chain (R group). The side chain is a prominent feature in the structure of glutamate, where it is an amino butanedioic acid group connected to the carbon atom of the carboxyl group. This group imparts polarity to glutamate, resulting in a negatively charged molecule. This property allows it to interact with specific receptors and ion channels, triggering downstream signalling cascades that regulate processes such as gene expression, synaptic plasticity and cell survival. Glutamate is the substrate for the vitamin K-dependent carboxylation reaction of coagulation factors, where glutamate is carboxylated to carboxyglutamate. The presence of carboxyglutamic acid enables certain coagulation factors to bind calcium ions, thereby facilitating their interaction with phospholipids on cell membranes during blood clotting. This modification ensures the proper function of these coagulation factors in the coagulation cascade reaction and facilitates the coagulation process.15
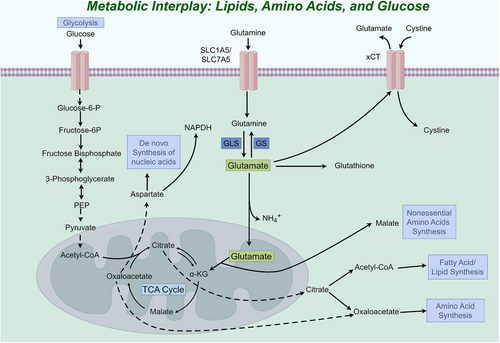
Within the nervous system, glutamate assumes multiple functions as a vital neurotransmitter, contributing to synaptic plasticity, modulation of glutamate receptors and synthesis of nitric oxide. Moreover, it plays a crucial role in fundamental processes such as cognitive abilities, memory formation, neuronal maturation and overall brain functionality.18 Neurons in the nervous system that release synaptic glutamate are called glutamatergic neurons. The role of glutamate in facilitating excitatory synaptic activation has been extensively demonstrated in academic research. When a neuron becomes excited, glutamate is released into the synaptic gap and binds to glutamate receptors on the neuron, triggering the transmission of nerve impulses. The excessive accumulation of glutamate leads to the demise of neurons through excitotoxic mechanisms.19 Synaptic function heavily relies on the presence of glutamate, which assumes a pivotal role in the formation of memories and the maintenance of optimal cognitive abilities. Its essentiality is underscored by the fact that it is necessary for normal cognitive function. It is involved in several key pathways within the brain, including cortical projections to the brainstem, the corticostriatal pathway connecting the prefrontal cortex to the striatum in the basal ganglia and thalamic projections to the cortex. Glutamate-mediated communication also occurs between pyramidal neurons in the cortex and between neurons in the cerebellum. However, glutamate receptors and transporters are not restricted to the nervous system, but are also present in other tissues and organs, including the lungs. Glutamate is associated with myocardial ischaemia/reperfusion injury and acute gastric mucosal injury.20, 21 Glutamate and glutamate signalling in the hypothalamus are involved in the regulation of appetite and energy balance, and alterations in glutamate signalling have been linked to the development of obesity and type 2 diabetes.22, 23 Glutamate and its receptors influence inflammation and cell fibrosis in diseases other than the nervous system.24 These studies have provided novel findings regarding the biology and pharmacology of glutamate, underscoring its potential therapeutic significance in diseases beyond the scope of neurological conditions. Chronic lung disease is characterised by metabolic reprogramming and metabolic disorders.25, 26 Thus, it may be beneficial to examine the role of glutamate signalling in chronic lung diseases. In brief, studying the functions of glutamate in the lung has important biological and pharmacological significance. In this article, we will review the current literature on glutamate metabolism, focusing on the dual function of glutamate and discussing its role in chronic lung diseases.
3 THE REGULATORY MECHANISM OF GLUTAMATE
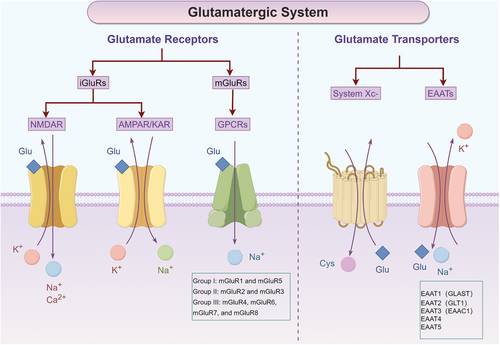
Precisely controlling intracellular and extracellular glutamate concentrations is essential for maintaining cellular homeostasis. Apart from glutamate metabolising enzymes, this regulation is primarily orchestrated by the precise function of glutamate transporters and glutamate receptors27 (Figure 2). Glutamate transporters are divided into two main groups, excitatory amino acid transporters (EAATs) and glutamate/cystine system (system Xc−).28, 29 There are five known subtypes of EAATs, which are typically designated as EAAT1 through EAAT5. In rodents, these transporters are also known as glutamate/aspartate transporter (GLAST), glutamate transporter 1 (GLT1), excitatory amino acid carrier 1 (EAAC1). EAAT4 and EAAT5 have the same nomenclature in both species.30, 31 Another important glutamate transporter, the system Xc−, is primarily responsible for the exchange of extracellular cystine with intracellular glutamate.32 Glutamate receptors are also important mechanisms for regulating glutamate concentration. Glutamate receptors are classified into two main types based on their function and structure: ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (mGluRs).13 The iGluRs have three subtypes, N-methyl-D-aspartate (NMDA) receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor) and kainate receptor. These iGluRs receptors are permeable to Ca2+, Na+ and K+ ion channels.33 The mGluRs are a family of G protein-coupled receptors (GPCRs) that respond to glutamate. Unlike iGluRs, mGluRs regulate intracellular signalling cascades by activating G proteins. There are eight subtypes of mGluRs, which can be categorised into three groups based on their sequence homology, pharmacology and signal transduction mechanisms. Group I mGluRs (mGluR1 and mGluR5) are associated with synaptic plasticity, neuronal development and neurodegenerative disorders. Group II mGluRs (mGluR2 and mGluR3) are involved in the regulation of neurotransmitter release, modulation of synaptic transmission and influence processes such as anxiety and cognition. Group III mGluRs (mGluR4, mGluR6, mGluR7 and mGluR8) participate in the regulation of neurotransmitter release, modulation of synaptic transmission and have effects on pain perception, addiction and motor coordination.34, 35
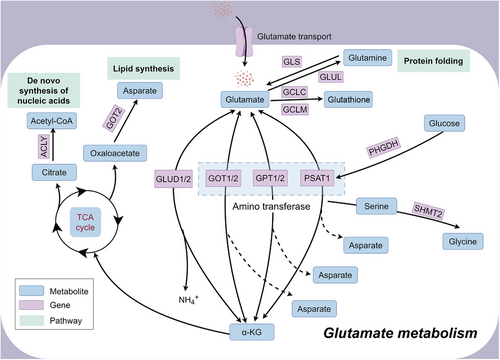
Glutamate metabolism is associated with the process of protein folding, de novo synthesis of nucleic acid and lipid synthesis (Figure 3). As a common neurotransmitter, glutamate is intricately involved in the pathogenesis of numerous neurological disorders, spanning from epileptic seizures and traumatic brain injuries to neurodegenerative diseases and psychiatric disorders. Its pivotal role extends across various pathophysiological mechanisms, including excitotoxicity, neuroinflammation and synaptic dysfunction. Abnormal glutamate release and excitotoxicity can contribute to epileptic seizures.34 Glutamate-induced excitotoxicity plays a significant role in promoting damage following an ischemic stroke. Ischaemia leads to abnormal glutamate release and impaired reuptake, promoting neuronal death.36 Alterations in glutamate neurotransmission can disrupt the balance within neural circuits involved in emotional regulation, reward processing and stress responses, contributing to mood disturbances and cognitive deficits characteristic of disorders such as depression, schizophrenia and bipolar disorder.37-39 In addition to neurological disorders, abnormalities in glutamate metabolism have been associated with disorders of other systems. Glutamate homeostasis is associated with diabetes, and the glutamate transporter EAAT2 is expressed on the cell membrane of pancreatic islet β-cells and physiologically controls extracellular glutamate concentration, alterations of which induce β-cell death leading to diabetes development.40 Hypothalamic glutamate signalling is associated with obesity and affects appetite regulation and energy homeostasis.41 Dysregulated glutamate metabolism and excitotoxicity have also been associated with the pathophysiology of heart failure, leading to myocardial damage and dysfunction, causing hypertension and impaired cardiovascular function.42 Glutamate receptors are expressed in articular cartilage and elevated glutamate levels in the bone tumour microenvironment increase cancerous bone pain.43 These associations emphasise the multiple physiological roles of glutamate beyond the central nervous system and highlight its importance in various disease processes in different organ systems.
4 GLUTAMATE METABOLISM IN CHRONIC LUNG DISEASES
The glutamate metabolism is involved in various chronic lung diseases (Figure 4). The study of glutamate in the lungs began in 1996. Research at that time showed for the first time the effect of NMDA receptors in the lungs. NMDA receptors are calcium-permeable glutamate receptors that are widely expressed at central excitatory synapses and elsewhere. The activation of NMDA receptors induced by glutamate influences several lung diseases. Upregulated of NMDA receptors could induce acute hyperpermeable lung oedema that could be completely suppressed by the NMDA receptor antagonist MK-801.44-46 Subsequent studies have also confirmed that NMDA receptor inhibitors can significantly reduce acute lung injury and pulmonary fibrosis induced by multiple factors like lipopolysaccharide, hyperoxia, bleomycin and mesenteric artery clamping/unclamping.47-51 The activation of NMDA receptors has also been shown to be a primary regulatory mechanism for airway inflammation and bronchial asthma hyperresponsiveness.52 In addition to NMDA receptors, there are other glutamate signalling pathways involved in the pathological process of chronic lung disease. Glutamine is a derivative of glutamate. Within the cell, glutamine can be converted from glutamate by the action of enzymes. Glutamine treatment can alleviate the pneumonia response induced by smoke inhalation in rats, and its protective mechanism may be related to inhibiting the inflammatory response and increasing the expression of heat shock proteins.53 The glutamine content in A549 cells changes most significantly with cigarette exposure.54 Glutamate receptors, specifically GPCRs, contribute to the development of pulmonary fibrosis primarily by stimulating pro-fibrotic activation of fibroblasts.55 Pulmonary hypertension is characterised by increased pulmonary vascular resistance and excessive proliferation of pulmonary arterial smooth muscle cells. GPCRs are important regulators of pulmonary vascular tone and pulmonary arterial smooth muscle cell phenotype. The dysregulation of GPCR expression and function contributes to the contraction and proliferation of smooth muscle cells in the pulmonary arteries. This imbalance disrupts pulmonary vascular tone, ultimately leading to the development of pulmonary hypertension.56-58 GPCRs exhibit differential regulation in the right ventricular myocardium, leading to adverse remodelling and functional impairment of the right ventricle.59 The glutamate transporter EAAT5 has been confirmed to be present in lung tissue.60 The glutamate transporter system Xc− is composed of the protein encoded by the SCL7A11 gene. SLC7A11 is involved in redox homeostasis, inflammation and immune regulation in lung tissue. Ferroptosis plays a crucial role in the pathophysiology of acute lung injury and COPD. SLC7A11 is an effective target for ferroptosis.61 The E3 ubiquitin ligase SOCS1 binds to and catalyses the ubiquitinated degradation of SLC7A11, thereby promoting ferroptosis in airway epithelial cells.62 SLC7A11 may play a role in the pathogenesis of pulmonary fibrosis by regulating redox balance, attenuating oxidative damage and modulating mechanisms associated with inflammation.24, 63
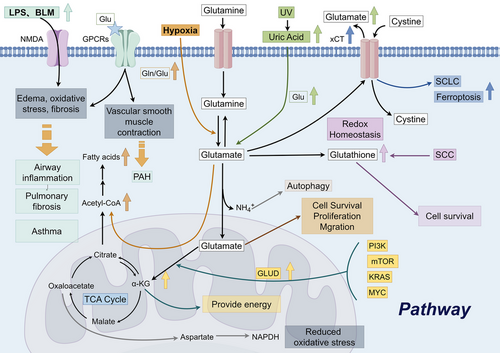
Chronic lung disease itself is not a cause of lung cancer, but individuals with chronic lung disease may have an increased risk of developing lung cancer. Persistent lung inflammation and tissue damage can result in aberrant cell proliferation and mutations, thereby raising the risk of lung cancer development. Tumour cells exhibit significant heterogeneity, with variations in metabolic mechanisms, molecular phenotypes and clinical characteristics even within the same cancer type. AAs are potential markers for cancer detection. The abnormal redistribution of AAs is the characteristic feature of the cellular metabolic changes in tumour cells.64, 65 Lung cancer exhibits elevated morbidity and mortality rates across various tumour types, and the lungs are a common site for the metastatic growth of various malignant tumours. Consequently, researchers strive to surmount the formidable health challenges posed by lung cancer. Lung cancer cells have a high demand for energy during rapid proliferation and active metabolism. Glutamate can provide energy through certain metabolic pathways to meet the growth and metabolic needs of lung cancer cells. Increased levels of glutamate contribute to cell survival, proliferation and migration.66, 67 Glutamate serves as a precursor for the prominent intracellular antioxidant glutathione, facilitating the maintenance of redox homeostasis within the cell. Increased glutamate uptake could help cystine uptake and glutathione synthesis, thereby promoting lung tumours.68 The excessive activation of phosphatidylinositol 3-kinase, protein kinase B (Akt), mammalian target of rapamycin, Kirsten rat sarcoma viral oncogene homolog genes, or the MYC proto-oncogene pathway in tumour cells promotes the metabolism of glutamate, resulting in the generation of α-KG through the actions of GLUD or transaminase enzymes. The elevated α-KG then enters the TCA cycle, providing a source of energy for tumour cells.69 Survival of lung squamous cell carcinoma also depends on both glucose and glutamine metabolism.70 Due to the significant role of glutamate metabolism in cellular growth and functioning, a diverse range of pharmaceutical agents have been formulated to disrupt intracellular glutamate metabolism, aiming to address various diseases.67 The system Xc− is considered a potential target for small-cell lung cancer treatment.71 Knocking out system Xc− renders cancer cells sensitive to oxidative stress, reducing lung metastasis of cancer cells.72 Glutamate and glutamine can be converted into each other under certain conditions. For glutamine to be fully utilised by the cell, it must be transported to the cell through a specific transporter and converted into glutamate by glutaminase. In many tumour cells, glutamine metabolism has been demonstrated to be dysregulated and is a necessary condition for the proliferation of most tumour cells.73 The above studies indicate that glutamate metabolism may play a vital role in lung cancer.
5 CONCLUSION
Glutamate assumes a critical role in AA metabolism, exerting a profound influence on a wide range of metabolic processes, signal transduction and essential nutritional functions. A growing body of evidence suggests that glutamate plays a crucial role in maintaining cellular homeostasis through autocrine and/or paracrine mechanisms. Within the central nervous system, glutamate serves as the primary excitatory neurotransmitter. Glutamate dysfunction has been implicated in the pathophysiology of several neuropsychiatric disorders, including schizophrenia, substance abuse and addiction, autism and depression. Numerous glutamate transporters and receptors present in various peripheral organs and tissues contribute significantly to the pathophysiological functions of the body, such as cardiopulmonary function, endocrine regulation and reproduction. These findings support the functional significance of glutamate as an essential AA. Glutamate has a significant impact on oxidative stress, inflammation and fibrosis processes in the lungs, thereby playing a critical part in the pathogenesis of chronic lung diseases. Changing the concentration of glutamate, including influencing its metabolism and modulating glutamate receptors and transporters, can have a mitigating effect on certain lung disorders. This article critically examines the significance of glutamate metabolism in the context of chronic lung diseases, while also contemplating potential avenues for future research.
AUTHOR CONTRIBUTIONS
Zhihou Guo and Furong Yan wrote and edited the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
All figures of this review are drawn by Figdraw. The work was supported by The National Natural Science Foundation of China (82200043), Joint Funds for the Innovation of Science and Technology, Fujian Provinece (2023Y9260), and Quanzhou City Science & Technology Program of China (2018N008S).
CONFLICT OF INTEREST STATEMENT
The authors have no relevant financial or non-financial interests to disclose.
ETHICS STATEMENT
Not applicable.




