Astrocytic functions and lipid metabolism: Correlations and therapeutic targets in Alzheimer's disease and glioblastoma
Abstract
Background
The brain is a central key organ of the body containing the second highest lipid content only after adipose tissue. Lipids as the main structural components of biological membranes play important roles in a vast number of biological processes within the brain such as energy homeostasis, material transport, signal transduction, neurogenesis and synaptogenesis, providing a balanced cellular environment required for proper functioning of brain cells. Lipids and their metabolism are of great physiological importance in view of the crucial roles of lipids in brain development and function. Astrocytes are the most abundant glial cells in the brain and involved in various processes including metabolic homeostasis, blood brain barrier maintenance, neuronal support and crosstalk.
Results
Disturbances in lipid metabolism and astrocytic functions may lead to pathological alterations associated with numerous neurological diseases like Alzheimer's disease (AD) recognised as the most frequent cause of dementia leading to major progressive memory and cognitive deficits as well as glioblastoma (GBM) known as the most aggressive malignant brain tumour with a poor prognosis.
Conclusions
Herein, we not only review the level and role of altered lipid metabolism in correlation with astrocytic function and astrocyte-neuron crosstalk in AD and GBM, but also discuss important lipid-related metabolites and proteins participating in possible mechanisms of pathologically dysregulated lipid metabolism, offering potential therapeutic targets in targeted molecular therapies for AD and GBM.
1 INTRODUCTION
The brain is a central and pivotal organ highly enriched in lipids (constituting 50–60% of brain dry weight),1 the major biomacromolecules characterised with poor water solubility and good solubility in non-polar organic solvent, and is regarded with the second highest lipid content next to the adipose tissue.2 Lipids are a class of fatty substances differing in overall structure, molecular weight, head group configuration, carbon–carbon bond formation and other factors, among which fatty acids (FAs), phospholipids (PLs), sphingolipids, sterol lipids and triglycerides (TGs) are the five main brain lipid classes,3 serving as basic structural components of biological membranes and participating in a broad variety of physiological events, including chemical energy generation and storage, substance transport, cellular signalling, neural differentiation, axonal regeneration, synaptogenesis, synaptic plasticity and brain development.4-14
The brain consists of neurons and non-neuronal cells such as glial and vascular epithelial cells, of which astrocytes represent the most abundant glial cells.15, 16 Astrocytes mediate diverse biological activities under physiological conditions, including structural and energy support for neurons,17, 18 neuronal development and maintenance,19, 20 formation, function and plasticity of synapses,21, 22 modulation of synaptic transmission,22 metabolomic homeostasis23 as well as integrity of the blood–brain barriers (BBBs)24, 25 which is a semipermeable membrane regulating solute exchange between blood and brain parenchyma to maintain CNS homeostasis and function and partially separating local lipid metabolism of the brain from that of the body.25-33 Apart from the well-known enzymatic capacity of glycogenesis and glycolysis,34-38 components of lipid metabolism also exists in astrocytes, providing membrane components for neurons and other glial cells39, 40 and playing fundamental roles in astrocyte function including membrane fluidity, energy generation and intercellular signalling. Emerging evidence has shown that astrocytic usage of lipids stored in droplets via mitochondrial β-oxidation fulfils crucial energy-providing and neuroprotective roles in the brain,18, 41 whereby disruption in lipid metabolism, structure and function of astrocytes may lead to pathogenic mechanisms underlying an array of neurological diseases (Figure 1).
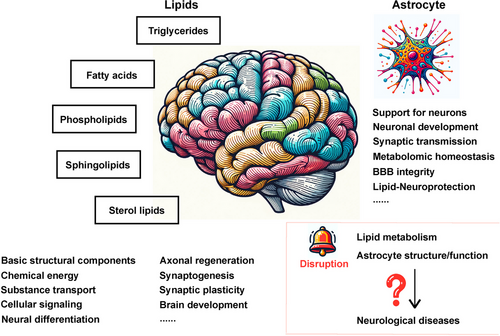
2 MAIN CLASSES OF LIPIDS IN THE BRAIN
2.1 Fatty acids
As one of the most well-known lipid class, fatty acids (FAs), the essential monomeric components of all lipids, account for almost 20% of the energy source through oxidation, for which astrocytes as the major provider of FA β-oxidation may be the essential place.42-44 Additionally, FAs can also be utilised by astrocytes for producing ketone bodies under particular conditions (e.g. ischemia), serving as a substrate for neuronal energy production-related tricarboxylic acid (TCA) cycle.45 FAs permeate the BBB through passive mechanisms, such as dissociation from albumin carriers, binding to the luminal membrane which belongs to endothelial cells, ATP-independent release and entrance into the cytosol. Additionally, they can traverse the BBB via protein-mediated transport, involving FA transport proteins (FATPs), FA translocase/CD36 (FAT/CD36), FA binding proteins (FABPs) and caveolin-1.46, 47 FAs can be further divided into unsaturated and saturated FAs (SFAs), from which the former subclass contains monounsaturated FAs (MUFAs) and polyunsaturated FAs (PUFAs), while the latter comprises palmitic acid, stearic acid and others.48, 49 PUFAs are highly enriched in the brain, with threefold to fourfold level over other tissues.50, 51 What is more, essential PUFAs play key roles in brain activity and development,52, 53 in which the ω−3 docosahexaenoic acid (DHA) are particularly involved in synaptogenesis, neurogenesis and neuroprotection in the brain.54-57
2.2 Phospholipids
As the most abundant component of major categories of membrane lipids,58, 59 phospholipids (PLs) generally consist of two hydrophobic tails of FAs differing in length and a backbone-attached hydrophilic phosphate group.60-62 PLs, which are synthesised in the mitochondria and endoplasmic reticulum (ER) tracing from diacylglycerol and phosphatidic acid (PA), spontaneously aggregate into the formation of bimolecular layers in aqueous environments on account of configuration and amphipathic propertys.63 PLs can be classified into glycerophospholipids and phosphosphingolipids, of which glycerophospholipids are the prominent glycerol-based class of lipid molecules which can be further subclassified into subtypes such as PA, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI) and phosphatidylserine (PS) on the basis of variation in hydrophilic head groups and participate in a variety of physiological activities in the brain.59, 64, 65 Moreover, fates of brain cells are influenced by exposure to different PLs, such as differentiation of neural cells into astrocytes was promoted and inhibited with PE and PC treatment, respectively.66
2.3 Sphingolipids
Sphingolipids containing sphingoid bases (also known as long-chain bases) and a set of aliphatic amino alcohols that includes sphingosine are mainly synthesised in ER. Sphingolipids comprise a large group of lipid molecules through compounding with different functional groups, such as ceramide (functional group of single hydrogen atoms) and sphingomyelin (SM) (functional groups including phosphocholine) with regards to structural composition, functioning as building blocks of membranes (e.g. lipid rafts)67 and playing fundamental roles in formation and regulation of synapse structure and function,68 cell recognition, signal transmission and inflammatory regulation of astrocytes.69-72 Besides, sphingolipid metabolites have also been discovered to exert regulatory roles in autophagy, cancer cell growth, response to DNA damage and inflammation.73-75
2.4 Sterol lipids
Sterol lipids include numerous organic molecules, of which cholesterol with four hydrocarbon rings is the main part. Cholesterol can be synthesised in ER by all nucleated cells, while over 70% of total body cholesterol are provided by the diet,76 namely the cholesterol absorbed in the gut transfers into the liver and then spreads through the body. What is noteworthy is that the brain, unlike other organs, makes its own cholesterol because of effective prevention of peripheral cholesterol exchange between brain tissue and plasma cholesterol-carrying lipoproteins by the BBB.77-79 In brain tissue, de novo synthesis of cholesterol is mainly performed in astrocytes which are considered as the main cholesterol producer in the brains,80 though the majority of sterol is synthesised in oligodendrocytes in developing brain and has an association with myelination81 and oligodendrocytes, besides, cholesterol can also be synthesised in many other cell typess.82-84 Apart from de novo synthesis,85 brain cells are able to acquire cholesterol from neighbouring cells through the absorption of cholesterol-laden lipoproteins (e.g. apolipoprotein E [ApoE]) in a receptor-mediated way,86, 87 in which lipoprotein synthesis for cholesterol transport occurs in astrocytes.88 With abundant existence in myelin and lipid membranes,81 cholesterol fulfils vital roles in the brain, including BBB integrity, organisation of lipid rafts (discrete microdomains present in the external leaflet of plasma membrane), regulation of cell membrane flexibility (through interaction with neighbouring PLs) and localisation and activity of diverse membrane proteins (e.g. membrane receptor and transporter proteins), axonal guidance, formation and maintenance of synapses and dendrites, synaptic membrane-related fluidity and ion channel function, glucose transport, intracellular signalling and other important neuronal functions.84, 89-103
2.5 Triglycerides
As the major form of FA deposition and the optimal form of FA triesters of glycerol, TGs are essential ingredients of glycerolipid synthesis by assembling with other glycerol moleculess.104 TGs mainly generated in the adipose tissue and liver can reach other tissues with the package into lipoproteins containing a hydrophilic exterior and a hydrophobic lipid core, including chylomicrons, very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL), very-high-density lipoproteins and high-density lipoproteins (HDL) only which can cross the BBBs.105-108 Additionally, APOE and apolipoprotein J, the most abundant apolipoproteins synthesised in astrocytes, serve as receptor ligands on HDL109-111 and play fundamental roles in lipid metabolism-associated structural support, enzyme activity and substrate deliverys.110, 112-114
3 ASTROCYTE-NEURON LIPID METABOLISM COUPLING
In humans, the brain representing, on average, merely 2% of total body weight consumes approximately and over 20% of energy substrates during quiet waking and diverse tasks, respectively,115, 116 which depends on relatively efficient metabolic coupling between astrocytes and neurons. Physiologically, astrocytes are considered primarily as glycolytic cells with a large enzymatic capacity for glycolysiss,115, 117, 118 whereas neurons are predominantly oxidative.119-121 Besides the glucose metabolism in which astrocytes participate in the delivery of blood-derived glucose to neurons as an obligatory energy fuel, glycogen storage and activity-dependent l-lactate production as a metabolic substrate for neurons during aerobic glycolysis,115, 122-124 astrocytes-neuron coupling of lipid metabolism has also been suggested to occur as a response to neuronal activity in protection of neurons from lipotoxicity.125, 126 This is a mechanism proposing that l-lactate-derived de novo synthesis of free FAs (FFAs) in overstimulated neurons is triggered during astrocyte-neuron l-lactate shuttle, resulting in excess FFAs in association to lipotoxicity-related reactive oxygen species (ROS) and lipid peroxidation chain reaction,127 peroxidised FAs with devastating effects127 are then transferred from hyperactive neurons to astrocytes via APOE-positive lipid particles, where they are directly stored in lipid droplets (LDs)125, 126, 128 which are dynamic organelles possessing a core of neutral lipids (e.g. cholesterol esters [CEs] and triacylglycerides), influencing FA breakdown for energy production129 and buffering excess FFAs to prevent lipid accumulation130 as well as utilised as an energy substrate in β-oxidation126 (Figure 2).
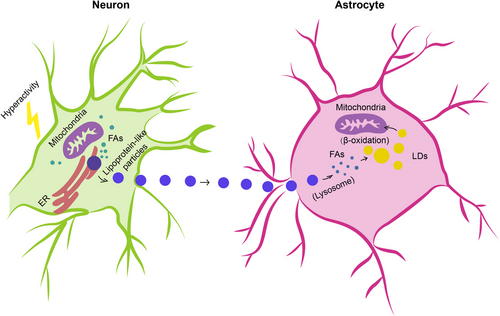
The dynamic interchange between astrocytes and neurons in lipid metabolism is essential for brain health, especially under stress or disease conditions. During neurodegenerative diseases or within the tumour microenvironment of glioblastoma (GBM), the lipid metabolism pathways in astrocytes undergo significant changes, impacting their ability to support neuronal survival.131-134 These conditions often lead to an imbalance in lipid homeostasis, resulting in increased neuronal susceptibility to lipid-induced damage. The altered lipid metabolism in astrocytes can contribute to the progression of neurodegenerative diseases by failing to adequately buffer the neuronal lipid overload or by inefficiently utilising lipids for energy production, thereby exacerbating neuronal dysfunction and cell death.133, 135, 136 Moreover, the astrocytic responses to neuronal signals may be compromised, leading to reduced neuroprotective actions and enhanced vulnerability of neurons to metabolic stress.135 In the context of disease, it is speculated that enhancing the astrocytic capacity to buffer and process FFAs might potentially mitigate neuronal damage and could lead to improved outcomes in neurodegenerative diseases and brain tumours.
4 PATHOLOGICAL LIPID ALTERATIONS AND LIPID-RELATED THERAPEUTIC TARGETS IN BRAIN DISEASES
4.1 Alzheimer's disease
With the worldwide increase in longevity, Alzheimer's disease (AD) has emerged as the most common form of senile dementia, rapidly evolving into a major health concern.137-139 This devastating, irreversible neurodegenerative disease is clinically manifested through memory loss, neuropsychiatric abnormalities, cognitive impairment, behavioural deficits and a progressive decline in self-care capacity.140-142 Pathologically, AD is characterise by the presence of extracellular amyloid-β (Aβ) plaques and intracellular neurofibrillary tangles (NFTs), composed of hyperphosphorylated tau protein.143-145 Embedded within this pathological framework are several influential hypotheses that further elucidate the mechanisms underpinning AD. The amyloid cascade hypothesis, for instance, posits that the aggregation of Aβ plaques is the primary event triggering the neurodegenerative cascade in AD.146, 147 This hypothesis is bolstered by genetic, biochemical and pathological evidence indicating that mutations in genes involved in Aβ metabolism, such as the amyloid precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2), lead to familial forms of AD characterised by increased Aβ production or aggregation. According to this hypothesis, Aβ plaques disrupt cell-to-cell communication, incite immune cell activation leading to inflammation and culminate in neuronal death and brain atrophy.146-150 Concurrently, the tau hypothesis centres on the intracellular accumulation of hyperphosphorylated tau protein, resulting in the formation of NFTs.151, 152 Tau, a protein integral to the stabilisation of microtubules in neurons, becomes pathologically phosphorylated in AD, losing its microtubule-binding capability and aggregating into NFTs.151-156 This disrupts neuronal transport systems and precipitates cell death. The interplay between Aβ accumulation and tau pathology, with evidence suggesting Aβ accumulation may precede and promote tau pathology, signifies a potential interdependence in AD pathogenesis. Moreover, the neuroinflammation hypothesis has garnered attention, advocating that neuroinflammation is not merely a consequence but a driving force in AD pathology. Chronic activation of the brain cells, such as microglia and astrocytes, in response to Aβ and tau pathology, engenders sustained neuroinflammatory responses that contribute significantly to neuronal damage and the progression of AD.157, 158
What is more, the accumulation of lipid granules in glia, besides notorious Aβ deposition and tau aggregates, was highlighted with the examination of Auguste Deter's brain (the first described AD patient), suggesting a possible involvement of perturbations of lipid metabolism in AD pathology.159, 160 Altered lipid metabolism has subsequently been identified as playing a significant role in the pathogenesis of AD.161-170
Recent AD pathology-related lipidome studies have demonstrated changes in content of numerous lipids (Table 1). Substantial differences in FA levels were observed in AD brain tissues,171, 172 including a decrease in levels of DHA present in frontal cortex grey matter173 and hippocampus174 to which damage correlates with impaired learning and memory,175 suggesting a dysregulation of FA metabolism and may potentially marking this neurodegenerative disease.176 Cholesterol accumulation observed in senile plaques and influenced brain regions from AD patients177 has been reported in association with region-specific synapse loss.178 A causal relationship between hypercholesterolemia and dysfunctional cholinergic system, cognitive impairments and pathology of amyloid and tau protein has been also demonstrated,179, 180 further supporting important roles of disturbed cholesterol metabolism in AD. What is more, detection of elevated CEs was performed in lipid raft-like mitochondria-associated ER membranes (MAMs)181 of which hyperactivity leads to cholesterol retention and synapse loss and correlates with cognitive deficits182 and in which accumulated cleaved products of APP cause mitochondrial dysfunction, interruption of cellular lipid homeostasis and membrane lipid alterations generally observed in AD pathogenesis.183, 184 Mitochondrial dysfunction, accompanied with increased oxidative stress, in neurons induces a lipid transfer to nearby astrocytes in which LDs accumulate, in turn, mitochondrial dysfunction in glial cells can be caused by accumulation of peroxidated lipids and oxidative stress, contributing to neurodegenerative processes.185-187 Growing evidence has supported nonnegligible roles of PLs and sphingolipids in AD pathogenesis and progression, with studies reporting that PLs and sphingolipids, together with acylglycerols, FAs and sterol lipids, present significant content changes in AD brain tissues.173, 188-194
| Lipids | Tissue | Changes in AD | References | ||
|---|---|---|---|---|---|
| Fatty acids | Omega-3 fatty acids | DHA | Brain; CSF; circulation | ↓ | 195-199 |
| MFG | ↑ | 198 | |||
| FCx | ↓ | 200 | |||
| EPA | Brain; circulation | ↓ | 199 | ||
| MFG | ↓ | 198 | |||
| DPA | Brain | ↑ | 201 | ||
| ALA | Plasma | ↑ | 202 | ||
| Omega-6 fatty acids | AA | Brain; CSF | ↑ | 196, 203, 204 | |
| MFG | ↓ | 198 | |||
| HPC | ↓ | 205 | |||
| LA | Brain; plasma | ↓ | 198, 206 | ||
| Saturated fatty acids | Brain; CSF | ↑ | 195 | ||
| Eicosanoids | PG | Brain | ↑ | 207 | |
| Phospholipids | PC | Total PC lipids | Brain | ↓ | 208 |
| PC-EPA | CSF | ↓ | 209 | ||
| PC-DHA | Plasma | ↓ | 210 | ||
| PC-EPA | Plasma | ↓ | 210 | ||
| PE | Total PE lipids | HPC | ↓ | 205 | |
| PE-SA | HPC | ↓ | 211 | ||
| PE-OA | HPC | ↓ | 211 | ||
| PE-AA | HPC | ↓ | 211 | ||
| PE-DHA | HPC | ↓ | 211 | ||
| PS | Total PS lipids | Occipital lobe; inferior parietal lobule | ↓ | 212 | |
| Sphingolipids | CM | Total CM lipids | Brain | ↑ | 213 |
| SM | Total SM lipids | CSF | ↓ | 214 | |
| Triglycerides | Total TG lipids | Serum | ↓ | 215 | |
| Polyunsaturated TG | Brain | ↓ | 216 | ||
| Sterol lipids | Cholesterol | Brain | ↓ | 217 | |
| Cholesterol precursors | Brain | ↑ | 217 | ||
| Total oxidised cholesterol | Brain | ↑ | s218 | ||
- Abbreviations: AA, arachidonic acid; ALA, alpha-linolenic acid; CM, ceramides; CSF, cerebral spinal fluid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; FCx, frontal cortex; HPC, hippocampus; LA, linoleic acid; MFG, medial frontal gyrus; OA, oleic acid; PC, phosphatidylcholine; PG, prostaglandin; PE, phosphatidylethanolamine; PS, phosphatidylserine; SM, sphingomyelin; SA, stearic acid; TG, triglyceride; ↑, increased from control; ↓, decreased from control.
4.1.1 APOE in AD
In comparison with early-onset familial AD, late-onset AD (LOAD) accounts for approximately 95% of all AD cases,219, 220 in which genetic predisposition, after aging, plays major roles in the onset of AD. As the strongest risk factor for LOAD, APOE is the main lipoprotein in the brain and plays pivotal roles in brain lipid metabolism, membrane remodelling and neuronal growth and repairs.221-225 APOE mainly produced by astrocytes is released into extracellular space where essential lipids (e.g. cholesterol) are delivered to neurons adopting APOE-bound cargo through APOE receptors expressed on the neuronal surfaces.221 In addition to the capacities of Aβ binding and influencing Aβ aggregation and clearance,223, 226 APOE participates in indirect regulation of Aβ metabolism through interactions with receptors (e.g. LDL receptor-related protein 1 [LRP1]).225, 227-232 Critical and isoform-specific role of APOE has also been demonstrated in formation of intraparenchymal Aβ deposits in APP transgenic mice.233-236 APOE exists with 3 different alleles namely APOEε2, APOEε3 and APOEε4, translating to three protein isoforms termed APOE2, APOE3 and APOE4, of which APOE4 present in approximately 14% of worldwide populations224, 237 is the most prevalent genetic risk factor for AD.238-241 A single amino acid difference between APOE3 and APOE4 (Cys 112 Arg) brings about a conformational change influencing the binding to Aβ, lipids and apolipoprotein receptors.242 APOEε2 considered as a protective genetic factor associated with reduced risk for AD and late age at onset238, 243 has been reported to orchestrate differences in lipidome and transcriptome profiles of postmortem AD brain.237, 244 Conversely, APOEε4 markedly elevates AD risk,238, 243 in which heterozygous and homozygous APOEε4 allele may increase AD risk by three and 12 times, respectively,242 accelerates disease course and worsens brain pathology.245-247 A correlation between APOE4 genotype and increased expression of Serpina3n, a gene expressed by astrocytes and considered as a strong marker of reactive and aged astrocytes in the brain,248, 249 has been reported with a possible contribution to the pathogenic role of APOE4 in AD.250 Higher APOE4 level in cerebral spinal fluid of AD patients compared with that of control individuals has been connected to accelerated Aβ oligomer accumulation.251 APOE4 may retard Aβ clearance and favour Aβ deposition via binding to Aβ after specific fragmentation.224, 242 APOE4 was reported to trap ATP-binding cassette transporters A1 (ABACA1) (a regulator of APOE4 lapidation in protection from lipid-poor ApoE4 aggregation) in late rather than recycling endosomes and alter ABACA1 membrane trafficking in astrocytes, which might result in reduced Aβ degradation.252 Insufficient Aβ clearance also affects accumulation in synaptic cleft, contributing to disruption of hippocampal long-term synaptic plasticity related to learning and memory abilities.253 APOE4 is internalised in APOE receptors such as LRP1 which is also a member of Aβ receptors including VLDL receptor and ApoE receptor 2 (APOER2).228 Additionally, APOE4-induced reduction of dendritic spine density in mice253, 254 is consistent with pathological changes (dendritic spine density reduction and synapse loss) observed in brain tissues from AD APOEε4 carriers.255 APOE4 causes widespread AD phenotypes-associated cellular and molecular alterations in brain cells derived from human induced pluripotent stem cells (iPSCs), among which increased Aβ secretion as well as impaired Aβ uptake and cholesterol accumulation occurred in neurons and astrocytes, respectively.256 Astrocytic lipid metabolism is influenced by APOE4,256, 257 in which increased FA unsaturation and LD accumulation were found in APOE4-expressing human iPSC-derived astrocytes, which can be restored to basal state through supplementation of culture medium with choline (a soluble PL precursor).257 Furthermore, APOE4 can also impair astrocyte-neuron coupling of FA metabolism via decreased FA sequestering in LDs, reduced LD transport efficiency and lowered FA oxidation, resulting in lipid accumulation in astrocytes and hippocampus, diminished abilities of astrocytes in neuronal lipid elimination and FA degradation, accelerated lipid dysregulation and increased AD risk.258
4.1.2 ACSBG1 and ACSL6 in AD
Cellular accumulation and activation of FAs either synthesised de novo or taken up from diets require the ATP-dependent reaction catalysed by acyl-CoA synthetases (ACSs), a family of enzymes initiating FA metabolism-related reactions through ligation to coenzyme A (CoA).259 ACS enzyme family contain various members differing in distribution and FA substrate preference,260 among which only two show specific enrichment in the brain, ACS bubblegum family member 1 (ACSBG1) and ACS long-chain family member 6 (ACSL6),261, 262 suggesting their potentially particular roles in modulation of brain FA metabolism. ACSBG1, almost exclusively expressed in astrocytes, have preferences for a wide range of substrates containing long-chain SFAs and unsaturated FAs.263, 264 ACSBG1 knockdown in vitro results in decreased ACS enzymatic activity and FA oxidation,264 indicating its participation in astrocytic FA oxidation, however, clear roles of ACSBG1 in brain function and/or dysfunction still remain poorly understood. ACSL6 showing high expression in the brain was reported to be downregulated in age-related neurodegenerative diseases265, 266 and in direct correlation with neurite outgrowths.267-271 With high substrate preference for DHA of which low levels are associated with AD pathophysiology,272 ACSL6 has been revealed with key roles in regulating DHA incorporation into neuronal membranes using Acsl6 deficient mice with significant reduction in DHA-containing PLs and impaired memory.273, 274 Critical roles of ACSL6 in brain DHA retention and neuroprotection are further supported by findings that ACSL6 depletion led to markedly reduced levels of brain membrane PL DHA, spatial memory deficits, hyperlocomotion, increased cholesterol biosynthesis and age-related neuroinflammation.275 What is noteworthy is that astrocyte-specific depletion had minimal influence on membrane lipid composition275 in consideration of ACSL6 enrichment in astrocytes,259, 276-280 possibly due to the expression of a DHA-non-preferring variant270, 281-286 and enrichment of Y-gate domain rather than DHA-preferring F-gate domain in astrocytes.270
4.1.3 ATAD3A in AD
ATPase family AAA-domain containing protein 3A (ATAD3A), a nuclear-encoded mitochondrial membrane-anchored protein belonging to the AAA+-ATPase protein family and simultaneously interacting with inner and outer mitochondrial membranes, is implicated in a variety of biological processes including stability maintenance of mitochondrial DNA, regulation of mitochondrial dynamics and cholesterol metabolism.287-289 ATAD3A deficiency led to neurodegenerative phenotypes in association with cholesterol elevation, downregulated expression of cholesterol metabolism-related genes,288 optic atrophy and axonal neuropathy.290 Oligomerisation and accumulation of ATAD3A at MAMs, lipid raft-like ER subdomain rich in SM and cholesterol291 and associated with diverse metabolic functions such as lipid metabolism, mitochondrial function and calcium homeostasiss,292-296 have been discovered in both mouse models and postmortem human brain tissues of AD.297 Aberrantly oligomerised ATAD3A leads to cholesterol accumulation via expression inhibition of cholesterol clearance-mediating cytochrome P450 (CYP450) family 46 subfamily A member 1 (CYP46A1) located on MAMs of which deficiency correlates with cholesterol disturbance, amyloid aggregation and cognitive impairments,298 AD-like MAM hyperconnectivity (e.g. impaired MAM integrity)296 as well as synapse loss.297 MAM-resident cholesterol imbalance facilitates amyloidogenic APP cleavage,184 in turn, retention of APP proteolytic fragments at MAMs interrupts cholesterol trafficking and homeostasis.299 Additionally, blocking ATAD3A oligomerisation by heterozygous knockout or pharmacological inhibition treated with DA1 peptide has been reported in causal relationship with cholesterol turnover normalisation, MAM integrity enhancement, APP processing suppression, synapse loss mitigation and ultimate reduction of AD-like neuropathology and cognitive impairments,297 further revealing a role of ATAD3A in AD pathology and suggesting a potential therapeutic strategy of retarding AD progression through manipulation of abnormal ATAD3A oligomerisation.
4.1.4 FoxO3 in AD
Forkhead box O transcription factor 3 (FoxO3) belonging to the forkhead box (FOX) family sharing an evolutionarily conserved forkhead DNA-binding domain composed of 80 to 100 amino acids300 and possessing single nucleotide polymorphisms (SNPs) associated with human longevity301, 302 functions as a mediator of biological processes promoting lifespan and preventing aging-related diseases,303, 304 of which alterations are involved in carcinoma, cardiovascular and neurodegenerative diseases.302, 305-308 FoxO3 plays a pivotal role in quiescence maintenance of neural stem cells (NSCs) in the brain, removal of which induces NSC differentiation and consequent NSC pool reduction.309-312 Apart from capacities for neuronal survival promotion or neuronal apoptosis mediation,313, 314 FoxO3 has also been shown with astrocyte proliferation controlling through inhibiting inflammatory cytokines (e.g. TNF-α and IL-1β) mediating reactive astrogliosis in neurodegenerative diseases.315-318 Conditional knockout of FoxO3 in astrocytes was reported to impair consumption of excess FAs319 which are cytotoxic and destructive to mitochondrial function.320 FoxO3 reduction in aged mice was found to be specific to the cortex rather than the hippocampus, where FoxO3 deficiency caused cortical astrogliosis and dysregulated lipid metabolism.319 In addition, lipid dysregulation, mitochondrial dysfunction together with Aβ uptake impairment were also observed in cultured astrocytes deficient in FoxO3, which could be reversed by astrocytic FoxO3 overexpression,319 potentially supporting the concept that FoxO3 elevation in astrocytes may retard or restore cortical astrogliosis and AD-associated impairments.
4.1.5 GSAP in AD
Under typical conditions, Aβ peptides as the products of body's cholesterol disturbance are cleaved from APP which may occur in two cellular pools, namely lipid raft-associated pool preferentially favouring APP cleavage by β- and γ-secretase as well as non-raft pools where cleavage is performed by α-secretase in a non-amyloidogenic pathway321 and rapidly eliminated to maintain normal Aβ levels.322 γ-Secretase activating protein (GSAP) was first reported for its regulatory roles in γ-secretase activity and specificity and its significant and selective enhancement of Aβ production through interactions with γ-secretase and APP carboxy-terminal fragment (APP-CTF).323 Significantly upregulated GSAP level has been demonstrated in both AD mouse models and postmortem brain tissues from AD patients.324-326 SNPs at the GSAP locus have been shown association with AD diagnosis,327, 328 of which one SNP was found to correlate with GSAP expression and AD risk.329 Genetic knockdown and pharmacological inhibition of GSAP suppress Aβ generation and deposition and tau phosphorylation in AD mouse models.323, 324, 330 Apart from the promotion of APP-CTF partitioning into Aβ production-favouring lipid rafts, GSAP has also been shown to be enriched in MAMs, an intracellular domain where amyloidogenic APP processing responsible for dysregulated lipid metabolism is performed.331, 332 GSAP depletion lowers APP-CTF accumulation in lipid rafts, reduces ER-mitochondrial contacts elevated in AD,332-335 and alters lipid profiles in a direction opposite to AD pathogenesis (e.g. GSAP depletion-raised levels of PE and PI showing consistent reduction in human AD brain).329, 336 What is more, interactions between GSAP and multiple components related to ER-associated degradation regulating mitochondrial function through MAM and participating in AD pathogenesis have also been revealed, further supporting crucial roles of GSAP in attenuating AD-associated pathogenic process.
Through lipidomic analysis, a detailed and comprehensive profile of lipid alterations in AD is offered, shedding light on the disease's underlying mechanisms and highlighting potential targets for intervention. The identification of significant changes in various lipid species, such as FAs, cholesterol, PLs and sphingolipids, in AD brain tissues, demonstrates the extensive involvement of lipid dysregulation in the disease. Notably, the decrease in DHA levels and the accumulation of cholesterol underscore the impact of lipid metabolism on AD's progression, linking these alterations to key pathological features like Aβ plaque formation and synaptic loss. The critical roles of APOE, especially the APOEε4 allele, are also emphasised in AD's pathogenesis through its influence on lipid metabolism. The involvement of enzymes and proteins such as ACSs and ATAD3A illustrates the potential of targeting lipid metabolic pathways in AD therapy. Lipidomic platforms facilitate early disease detection through identifying biomarkers and aiding in the development of preventive and therapeutic strategies. The advancements in lipidomics offer promising avenues for improving AD understanding, enhancing early diagnosis and developing targeted therapies, underscoring the significance of lipid regulation in AD treatment approaches.
The discovery of novel drug targets, such as genetic factors like APOEε4 and enzymes involved in lipid metabolism disruptions (e.g. ACSBG1, ACSL6 and ATAD3A), opens avenues for targeted therapies that aim to modify disease progression or alleviate symptoms.176, 223, 337 These potential treatments, including those targeting the stabilisation of lipid homeostasis and mitochondrial function, present a promising strategy given the important roles of altered lipid metabolism in AD pathology (Table 2).338, 339 However, the development of effective treatments is complicated by AD's multifactorial nature, involving a complex interplay of genetic, molecular and environmental factors.340 The challenges extend to drug delivery, particularly across the BBB, and the variability in patient response due to genetic and phenotypic heterogeneity among AD patients.341, 342 Current therapies offer limited effectiveness, primarily providing symptomatic relief without significantly altering the disease course.343 Moreover, therapeutic interventions targeting the central nervous system often carry significant side effects, and the effectiveness of many potential therapies depends on early intervention, which is hampered by current limitations in early diagnosis.344, 345 Therefore, while the prospects for innovative treatments targeting specific molecular disruptions in AD are promising, overcoming the multifaceted challenges requires a comprehensive approach that integrates advances in genetics, molecular biology and pharmacology with improvements in diagnostic methodologies to enable timely therapeutic interventions.346, 347
| NCT number | Disease | Lipid | Status | Phases | Enrolment | Study type | URL |
|---|---|---|---|---|---|---|---|
| NCT05218018 | AD | Triglyceride | Completed | Not applicable | 30 | Interventional | https://ClinicalTrials.gov/show/NCT05218018 |
| NCT00303277 | AD | Cholesterol | Completed | Phase 4 | 35 | Interventional | https://ClinicalTrials.gov/show/NCT00303277 |
| NCT02719327 | AD | Eicosapentaenoic acid | Completed | Phase 2/Phase 3 | 131 | Interventional | https://ClinicalTrials.gov/show/NCT02719327 |
| NCT00053599 | AD | Cholesterol | Completed | Phase 3 | 400 | Interventional | https://ClinicalTrials.gov/show/NCT00053599 |
| NCT00939822 | AD | Cholesterol | Completed | Phase 2 | 88 | Interventional | https://ClinicalTrials.gov/show/NCT00939822 |
| NCT00486044 | AD | Cholesterol | Completed | Phase 2 | 103 | Interventional | https://ClinicalTrials.gov/show/NCT00486044 |
| NCT00151502 | AD | Cholesterol | Completed | Phase 3 | 600 | Interventional | https://ClinicalTrials.gov/show/NCT00151502 |
| NCT01142336 | AD | Cholesterol | Completed | Phase 4 | 49 | Interventional | https://ClinicalTrials.gov/show/NCT01142336 |
| NCT02029573 | GBM | Cholesterol | Completed | Phase 2 | 36 | Interventional | https://ClinicalTrials.gov/show/NCT02029573 |
4.2 Glioblastoma
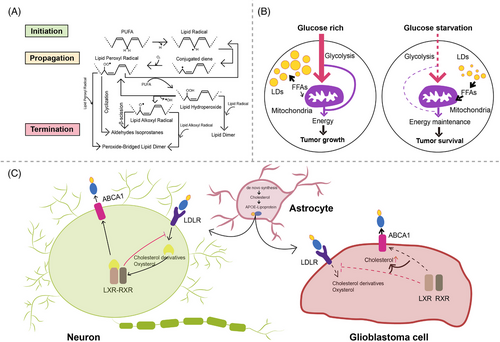
Glioma as a malignant primary brain tumour originating from astrocytes or other glial cells accounts for approximately 80% of all malignant brain tumours,348 of which GBM is the most aggressive type of brain tumour known with a 5-year survival rate below 5%.349-351 Metabolic reprogramming has been recognised as a fundamental hallmark for carcinogenesis and progression of multiple tumours including GBMs,352-354 through which tumour cells meet the high-energy demands of rapid proliferation.355 Except for the representative metabolic feature named the Warburg effect, a phenomenon in which GBM cells rely on glycolysis for energy production under oxygen-sufficient and oxygen-insufficient conditions,353, 355-357 GBM cells can also be fuelled by FA oxidation (FAO) as an alternative crucial energy resource to meet high-energy consumption in GBM aggressiveness,358-362 of which inhibition negatively impacted GBM proliferation and progression.363
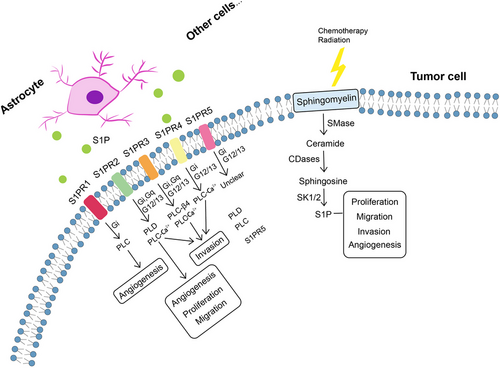
Oxidation of FAs is achieved by two major pathways, namely enzymatic oxidation mediated by peroxidases (e.g. cyclooxygenase, CYP450, lipoxygenase and phospholipase A2)364 as well as non-enzymatic self-catalysed peroxidation (Figure 3A) of which 4-hydroxynonenal (4-HNE) is an end-product showing elevated expression proportional to the grade of brain tumour malignancy.365-367 Moreover, lipid metabolism reprogramming in association with numerous pathophysiological processes such as tumour proliferation and development368-373 has been further evidenced with the observation of large amounts of LDs in GBM374-376 and other tumours.377-384 Neutral lipid core of a single LD includes cholesteryl esters and TGs composed of glycerol molecules with triple hydroxyl groups esterified by FAs.385-388 TGs have been demonstrated to serve as an important energy reservoir for supporting GBM cell survival, in which LDs were rapidly broken down by GBM cells via autophagy, a pivotal cellular process degrading damaged organelles and protein aggregates and recycling nutrients via hydrolysis of cytoplasmic components to ultimately maintain cellular homeostasis,389-392 to release stored FAs for producing energy upon energetic stress like glucose deprivation (Figure 3B), in turn, inhibition of FAO or autophagy led to LD retention and significant potentiation of GBM cell death,393 suggesting that LDs may play critical roles in regulating GBM growth and limitation of LD usage might be indispensable in GBM treatment. What is more, cholesterol metabolism in GBM is different from that in healthy brain tissues where nearly all brain cholesterol is synthesised de novo.394-396 Contrary to normal astrocytes mainly synthesising cholesterol from glucose or glutamine397-399 and converting excess cholesterol to oxysterol as an endogenous ligand of liver X receptors (LXRs) to consequently trigger efflux of surplus cholesterol via ABCA1 and suppression of cholesterol uptake by LDL receptors (LDLRs),400-404 GBM cells are insufficient to de novo synthesise cholesterol and thus dependent on exogenously supplied cholesterol for survival through upregulated LDLR expression394, 405 (Figure 3C), in which LXR agonists could induce GBM cell death by lowering intracellular cholesterol content via ABCA1-dependent cholesterol efflux and LDLR inhibition.394 Additionally, intracellular cholesterol level has been revealed to be involved in resistance against GBM cell death induced by temozolomide (TMZ), a BBB penetrant chemotherapy agent currently used in the standard therapy for patients with GBM.406, 407 Furthermore, SMs, an important group of PLs in cell membranes, together with their hydrolysis by sphingomyelinases (SMase) are crucial to effects of radio- and chemotherapy.408-411 Ceramides which are generated by SMase-mediated SM hydrolysis caused by TMZ and radiation can induce cell apoptosis,412-414 which can be evaded through conversion of ceramides to sphingosine-1-phosphate (S1P) (Figure 4)415-417 linked to tumour grade and implicated in GBM aggressive phenotypes.413, 418
4.2.1 S1PRs in GBM
GBM cells utilise exogenous source of S1P synthesised and exported by astrocytes and neuronal cells419 and endogenous S1P production420 for tumour progression. Involvement of S1P in tumour growth, migration, invasion, survival and angiogenesis421-424 is specifically mediated by the family of G-protein coupled receptors named S1P receptors (S1PRs, S1PR1–S1PR5).425-430 S1PR1, S1PR2, S1PR3 and S1PR5 are expressed in human GBM cells,431-433 and elevated levels of S1PR1, S1PR2 and S1PR3 have been detected in brain tissues from GBM patients compared with healthy tissues, while only S1PR1 and S1PR2 showed significant association with GBM survival rates.431, 432 S1PRs are essential for mediating diverse S1P functions, whereas orientations in which they influence cell phenotypes still remain unclear. S1PR1 inhibition was reported to promote GBM cell proliferation, which collides with studies suggesting increased GBM proliferation by S1PR1–3, of which S1PR1 showed the strongest effects.432, 434 S1PR2 was shown to both reduce GBM migration through Rho/Rho kinase signalling pathway and participate in promoting GBM invasion.435, 436 In addition, S1PR5 has also been identified as an independent prognostic factor of GBM patients’ survival, aligning with reported role of S1PR5 in proliferation promotion.434, 437 Pharmacologically altered S1PR expression by fingolimod (FTY720), a sphingosine analogue leading to S1PR1 internalisation, has been revealed to suppress astrocyte activation and change astrocytic secretion of C-X-C motif chemokine 5 (CXCL5) known to promote GBM proliferation and migration.438-440 Furthermore, functions of individual S1P receptor subtypes are dependent upon activation of diverse downstream effector proteins, especially coupling to different G-proteins,429 such as binding of S1PR1, S1PR2 and S1PR5 with Gi, activation of Gq by S1PR2 and S1PR3 as well as signalling of S1PR2, S1PR3 and S1PR5 via G12/13 (Figure 3),441 which alters signalling of phospholipases (particularly phospholipase C (PLC) cleaving proximal phosphodiester bonds of glycerophospholipids in production of phosphorylated headgroups and diacylglycerols429, 430) and further activates downstream signalling molecules (e.g. extracellular signal-regulated kinase, phosphoinositide 3-kinase and mitogen-activated protein kinase) (Table 3). What is noteworthy is that a S1PR-targeted liposomal drug delivery system, named S1P/JS-K/Lipo, capable of blood–brain tumour barrier penetration and enhanced tumour-targeted delivery has recently been described, efficiently delivering a nitric oxide prodrug (JS-K, O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl) piperazin-1-yl] diazen-1-ium-1,2-diolate) to GBM tissues via specific interactions with S1PRs highly expressed on GBM cells,442 representing a promising targeted approach for GBM therapy.
| Models | Involved S1PRs | Signalling pathways | Findings | References |
|---|---|---|---|---|
|
LN18 GBM cells; U87MG GBM cells |
S1PR1 ↑ S1PR2 ↑ S1PR3 ↑ |
PI3K/AKT1 pathway |
Demonstrated association between S1P1 and S1P2 with GBM patient's survival. S1PR1/2 inhibition reduces GBM migration. |
443 |
| U373MG GBM cells |
S1PR1 ↑ S1PR2 ↑ S1PR3 ↑ |
MAPK/ERK and PI3Kβ pathway | S1P promotes glioma cell proliferation. | 444 |
|
U373MG GBM cells; GBM6 cells; GBM12 cells |
S1PR2 | MEK1/2 and Rho/ROCK | S1P induces mRNA and protein expression of PAI-1 and uPAR, which are important for GBM invasiveness. | 445 |
|
U373MG GBM cells; U118MG GBM cells |
S1PR1↑ S1PR2 S1PR3 |
MAPK-ERK Rho/ROCK |
S1PR, S1PR2 and S1PR3 all positively contribute to S1P-stimulated glioma cell proliferation, of which S1PR1 makes the major contribution. | 446 |
| C6 glioma cells | S1PR2 |
MAPK/ERK, PKC, PLC, PLD and Ca2+ signalling |
S1PRs are linked to at least two signalling pathways (i.e. PTX-sensitive Gi/Go–protein pathway and toxin-insensitive Gq/G11–PLC pathway). | 447 |
|
C6 glioma cells; 1321-N1 astrocytoma cells |
S1PR2 | PI3K/Cdc42/p38MAPK and PI3K/Rac1/JNK | S1PR2 mediates S1P-induced negative regulation of glioma cell migration. | ADDIN448 |
|
U373MG GBM cells; U87MG GBM cells; M059K cells; U-1242 cells; A172 cells |
S1PR1 ↑ S1PR2 ↑ S1PR3 ↑ |
MAPK/ERK and PI3K | S1P potently enhances glioma cell motility by signalling through coupling of S1PRs to Gi proteins. | 449 |
|
T98G glioma cells; G112 glioma cells |
S1P1, S1P2, S1P3 and S1P5 | PTEN/AKT/Egr |
S1PR1 is a significant prognostic factor for glioma. Downregulated S1PR1 expression increases glioma cell proliferation and enhances glioma malignancy. |
450 |
|
Human GBM specimens; U87 glioma cells; U251 glioma cells; T98G glioma cells; G112 glioma cells |
S1PR1↓ |
Downregulated S1PR1 expression in GBM patients with a poor survival. S1PR1 signalling negatively controls glioma cell proliferation. |
451 |
- Abbreviations: AKT, v-akt murine thymoma viral oncogene homolog; Cdc42, cell division control protein 42 homolog; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; PLD, phospholipase D; PTEN, phosphatase and tensin homolog; PTX, pertussis toxin; Rac1, Ras-related C3 botulinum toxin substrate 1; ROCK, Rho-associated protein kinase.
4.2.2 FABP7 in GBM
Fatty acid binding protein 7 (FABP7), a member of the multi-gene FABP family comprised of structurally related proteins with expression patterns specific to cell, tissue and development, binds to very-long-chain PUFAs such as DHA with high affinity.452, 453 FABP7 abundant in astrocytes454-456 is a lipid chaperone mediating cellular uptake, intracellular trafficking and subsequent oxidation of FAs, whose expression was reported to be elevated in GBM and GBM stem-like cells forming neurospheres and might accounting for GBM aggressiveness457, 458 and recurrence as well as associated with proliferation, migration and invasion of GBM cells, GBM histology and reduced survival time.459-464 Under metabolic stresses (e.g. hypoxia), FAs are stored as LDs and subsequently oxidised in a FABP-dependent manner for energy production in GBM cells.465 Slow-cycling cells (SCCs), a subpopulation of GBM cells preferentially utilising mitochondrial oxidative phosphorylation (OXPHOS), showing elevated lipid contents specifically metabolised under glucose deprivation and displaying enhanced capabilities of migration, invasion and chemoresistance, have been revealed with the characterisation of higher FABP expression and larger LD amounts in cultured conditions of normal oxygen levels or nutrients.466 Additionally, resistance of SCCs against deprived glucose or inhibited glycolysis could be restrained by FA uptake blocking via genetic deletion or pharmacological inhibition of FABP7.466 Moreover, promotion effects of FABP7 on GBM cell migration can be mitigated with DHA supplementation through specific and dramatic inhibition of DHA supplementation in culture medium on plasma membrane lipid order of FABP7-expressing GBM cells which positively correlates with GBM cell migration as well as DHA supplementation-mediated disruption of nanodomains formed by FABP7 on GBM cell membranes,467 further suggesting a critical role of FABP7 in lipid metabolism in GBM cells.
4.3 SCD in GBM
Stearoyl-CoA desaturase (SCD) is an ER-localised delta-9 FA desaturase forming carbon–carbon double bonds at the 9th to 10th position from the COOH-terminus of SFAs, stearic acid and palmitic acid and thus generating MUFAs, oleic acid and palmitoleic acid, respectively,468, 469 whose expression correlates with the ratio of MUFA to SFA in which a disequilibrium contributes to alterations in cell growth and differentiation.468-471 SCD has 4 isoforms in mice (SCD1, SCD2, SCD3 and SCD4), while only two paralogs are expressed in human, namely SCD sharing approximately 85% amino acid identity with mouse SCDs and SCD5 unique to primates.470, 472 SCD has been described as a hypermethylated gene member contributing to the CpG island methylator phenotype which defines a distinct glioma subgroup.473 SCD expression in GBM, in contrast to SCD upregulation often observed in multiple human tumours,474-477 was reported to be lower than normal brain tissues because of hypermethylation and monoallelic deletion together with phosphatase and tensin homolog frequently deleted in GBM478 in a subset of GBM patients.479 In addition, GBM cells without epigenetic and genetic changes mentioned above were revealed to express elevated SCD levels on which tumour cells rely for their survival.479 SCD inhibition by CAY10566, an inhibitor with a modest BBB penetration ability, has been demonstrated to not only significantly suppress intracranial GBM growth, but also obviously affect tumour vasculature including nearly complete blocking of intratumoural bleeding and possible normalisation of blood vessels, potentially allowing enhanced delivery of combinedly used antitumour drugs such as TMZ.479
The exploration of GBM lipid metabolism, through studies on FA oxidation, LDs accumulation and cholesterol metabolism alterations, suggests lipidomic analysis as a valuable tool for the early detection of GBM and the identification of new therapeutic targets. Markers like elevated 4-HNE levels and abundant LDs in GBM cells indicate tumour aggressiveness and highlight metabolic pathways ripe for intervention. GBM's reliance on external cholesterol sources, along with the important roles of proteins such as FABP7 and SCD, reveal exploitable metabolic vulnerabilities for developing treatment strategies (Table 2). Additionally, sphingolipids, especially S1P and its receptors, emerge as important components in tumour progression, serving both as biomarkers and potential therapeutic targets. Advancements in lipidomic platforms that allow for precise sphingolipid measurement might facilitate more effective GBM detection and therapy assessment.
5 CONCLUSIONS
The brain is highly enriched in lipids where they are crucial for multiple physiological processes ranging from maintenance of structural integrity and metabolic homeostasis to brain function and development. Metabolism of lipids is a complicated process in which a wide range of lipid-related effector proteins are involved and whose alteration is strongly associated with brain dysfunctions and diseases such as AD and GBM. In this review, we throw light upon basic classes of lipids including FAs, PLs, sphingolipids, sterol lipids and TGs, of which dysregulated metabolism can be regarded as disease biomarkers. We also briefly discuss the role of lipids within the brain and altered lipid profile correlated with astrocytic function and astrocyte-neuron crosstalk in AD and GBM. Moreover, we have discussed lipid-related metabolites and proteins critical for disease-associated lipid dyshomeostasis and how these proteins together with lipids in correlation with astrocytic functions modulate disease pathogenesis and development, enlightening their therapeutic potential in preventing onset and progression of AD and GBM. However, there are still several lipids whose association with AD and GBM and availability as clinically valuable biomarkers for disease detection at early stages need further evaluation, which can be performed by newly developed and improved techniques of gradually matured lipidomic platforms. What is more, there remains much to be discovered about benefits and risks of manipulation of compounds affecting effector proteins involved in lipid metabolism, and further characterisation of pathways in which important lipid-related proteins participate along with clinical studies will aid the understanding of pathogenesis mechanisms behind AD and GBM and identification of novel therapeutic targets to help ameliorate disease courses, facilitate disease treatments and consequently benefit patients.
AUTHOR CONTRIBUTIONS
X. Z. takes primary responsibility for the paper. X. Z. drafted the manuscript. R. R. G., Y. W. Z., L. K. Y., T. C. Z., X. R. S., W. W. G., L. Y. and S. J. P. participated in the literature search and manuscript writing. All the authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
FUNDING INFORMATION
Not applicable.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




