Unveiling a novel cellular stress response mechanism to photodamage
Abstract
Ultraviolet (UV) radiation, a component of sunlight, holds both advantageous anddetrimental effects on human health. While shorter wavelengths of UV radiationaid in melanin and vitamin D synthesis, longer wavelengths pose risks like skincancer and premature aging due to DNA damage. To combat such stress, cellsemploy various mechanisms, including the heat shock response (HSR). Activation of this response involves a highly regulated transcriptional processorchestrated by heat shock factors (HSFs). While HSF1 has been observed as a keytranscription factor for HSR, other HSFs are also found to be associated withdiverse cellular functions, including stress responses. Here, we discuss arecent study by Feng et al., published in Clinical and Translational Medicine, shedding light on the novel function of HSF4 in regulating inflammation and senescence following UV exposure. The researchers observed acomplex of HSF4 and the cofactor COIL (Coilin) at R-loops–aberrant DNA-RNAhybrid structures arising from UV-induced DNA damage in human skin cells. Inthe study, they proposed the HSF4-COIL complex at R-loops as a potential therapeutic target to mitigate UV-induced skin damage.
Sunlight is a mixture of electromagnetic waves and is one major source of energy for Earth's life. Its spectrum spans low-energy radio waves to high-energy gamma rays, and these diverse wavelengths orchestrate a symphony of cellular processes, underpinning life's intricacies. For example, visible light is essential for photosynthesis, a chemical process in chlorophyll-containing organisms to yield organic compounds and oxygen. This visible light also governs circadian rhythms and hormonal equilibrium in many organisms, including humans. Sunlight also contains ultraviolet (UV) radiation, which can have both beneficial and detrimental effects on organisms. Shorter wavelength UVB impacts the skin and overall health through the induction of melanin and vitamin D synthesis, although longer exposure can cause sunburns. The longer wavelength UVA, however, infiltrates deeper into the skin and can result in DNA damage, which can have long-lasting consequences including increased risk for skin cancers and premature skin ageing.1, 2 According to the World Health Organization (WHO), every year, around 2–3 million people worldwide are diagnosed with different skin cancers which are often associated with UV toxicity. This alarming statistic drives researchers to investigate the cellular response to UV exposure for the development of better therapeutic interventions that mitigate the detrimental impacts of UV radiation on human health.
Cells have a remarkable ability to respond to various forms of stress, adopting an array of strategies to counteract environmental insults and maintain their internal homeostatic environment. However, when the damage inflicted is beyond repair, cells engage in programmed cell death pathways as a last resort. In the context of stress impacting protein stability, cells enact a defence mechanism involving the production of specific proteins, including heat shock proteins, which are mainly known for their protein-refolding (i.e. chaperone) activity.3 The orchestration of this process occurs at the transcriptional level, regulated by the heat shock factor (HSF) proteins that function as key transcription factors. Various stressors activate HSFs to bind to a specific DNA element present at the promoter of their target genes, facilitating the activation of their expression (Figure 1). Among HSFs, HSF1 is the most well-studied transcription factor, which plays critical roles in the response to multiple stressors, including heat, oxidative damage, and mitochondrial stress. HSF1 has also been shown to be essential for maintaining protein homeostasis and cellular resilience in the face of stress-induced challenges. It not only regulates the expression of chaperone proteins critical for protein folding and cellular recovery, but also contributes to a broader transcriptional program including cellular immune response, cell growth and cell death pathways.4 Unlike HSF1, HSF2 was originally thought to be a nonstress-responsive transcription factor playing roles in other functions such as reproductive health; however, several studies have now shown that it can form a heterotrimer with HSF1 in response to stress and drive the transcription of heat-responsive genes.5 HSF3 is commonly studied as an avian HSF, although one study has identified a role for a mouse HSF3 to regulate non-classical heat-shock genes to protect cells from stress.6 Finally, HSF4 has been found to play an important role in vertebrate lens cells for development and maintenance by controlling the expression of crystallin proteins vital for lens transparency and function. Mutations in HSF4 have been found to cause problems like cataracts, which make the lens cloudy and affect the ability to see clearly.7
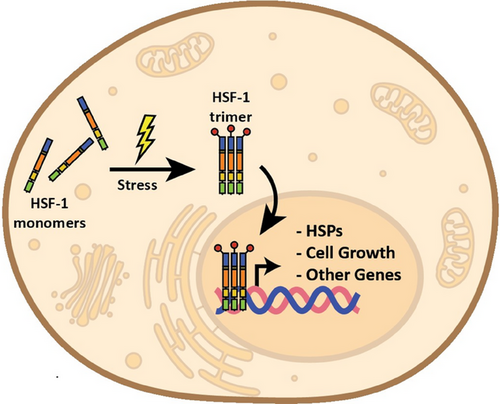
In this context, Zhang et al. in their recent publication in Clinical and Translational Medicine have shed light on a previously unexplored facet of the regulation of cellular stress responses by HSF4. In this study, they provide a novel mechanism for HSF4 to regulate transcription in skin cells in response to UV stimuli along with a cofactor, COIL (coilin), which had previously been demonstrated to accumulate at sites of DNA damage, although the exact molecular function had not yet been revealed until this study. DNA damage can lead to transcriptional stress by forming inappropriate DNA-RNA hybrid structures called R-loops (Figure 2A), which can introduce a chronic inflammatory response if not resolved. Unresolved R-loops can cause further DNA damage and genomic instability, leading to the onset of autoimmune disorders, cancer, and premature ageing.8, 9 In this study, authors observed that UV stimulation results in a prolonged inflammatory response due to the increased number of R-loops. Further investigation into the molecular mechanism revealed the formation of a novel complex of HSF4 and COIL, which they argue accumulates at R-loop sites to drive the induction of genes involved in inflammation and senescence. This complex gene regulation required both HSF4 and COIL as overexpression of any single transcription factor was not sufficient to drive this transcriptional response (Figure 2B).
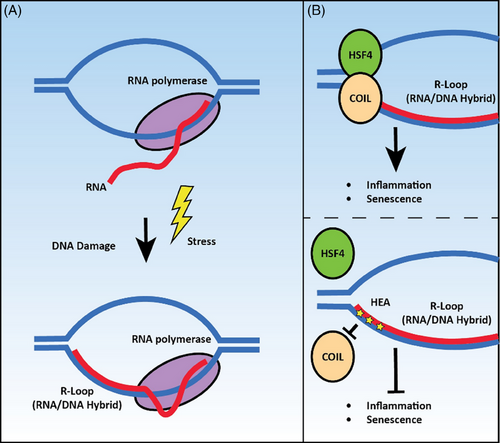
The observations in this study established the association of the HSF4-COIL complex with the R-loop structure as a potential drug target to rescue skin cells from the pathological damage caused by UV radiation. Indeed, the authors have screened several natural nucleotide analogues and identified N6-(2-hydroxyethyl)-adenosine as a promising drug that can successfully compete with R-loops for binding to COIL (Figure 2B). This limits the interaction of the HSF4-COIL complex with R-loops to significantly inhibit the expression of inflammation and ageing-related genes in cells and reduce pathological phenotypes in mouse models. Overall, this study provides us with a wealth of novel insights. First, it identified a novel HSF4-COIL complex that can drive the pathological response to UV stress. Not only did these studies characterize a unique molecular mechanism for driving transcriptional responses to stress, but also highlighted the important fact that HSFs are canonical stress response transcription factors. These stress responses may not always be positive and can have pathological outcomes. Similarly, other HSFs can drive pathology, such as HSF1's well-studied role in cancer.10 In addition, this study mechanistically placed HSF4-COIL at R-loops and identified specific genetic targets that can promote inflammatory and senescent gene expression and a potential therapeutic strategy to mitigate this response. These findings are not only limited to fighting against DNA damage associated with UV exposure but could potentially be extrapolated to illuminate responses to other forms of DNA damage, thereby enhancing our strategies in protecting cellular health and combating a spectrum of stressors that challenge DNA integrity.
AUTHOR CONTRIBUTIONS
N.D. prepared the manuscript; G.G. prepared the figures; and R.H.S. edited the manuscript.
ACKNOWLEDGMENTS
National Institute on Aging: R00AG065200 to Ryo Higuchi-Sanabria and T32AG052374 to Gilberto Garcia. Larry L Hillblom Foundation Grant 2022-A-010-SUP to Ryo Higuchi-Sanabria.
ETHICS STATEMENT
Not applicable.




