Deubiquitination of Abelson tyrosine kinase: A novel regulatory mechanism targeting non-small cell lung cancer glycolysis
Abstract
In this commentary, we briefly introduce the importance of metabolic reprogramming in non-small celllung cancer (NSCLC) and the role of aberrant glycolysis processes intumorigenesis and metastasis. We further summarize the regulation of deubiquitylation modification on glycolysis-related kinases in this system. Andwe discusse the major discovery the research by He Yuanming et al. published in Clinical and Translational Medicine.Their study demonstrates targeting the USP7-c-Abl-HK2 axis represents a promising therapeutic strategt for NSCLC.
Lung cancer is a prominent malignancy significantly impacting human health and represents a crucial contributor to global cancer-related mortality, with an annual increase of 1.6 million new cases and 1.38 million deaths. Non-small-cell lung cancer (NSCLC) constitutes the predominant pathological subtype, accounting for about 80%–85% of all diagnosed cases.1 With the development and clinical application of new therapeutic techniques, including surgery, neoadjuvant chemotherapy, targeted therapy and immunotherapy, the quality of life of patients with NSCLC has been effectively improved. Nevertheless, due to the heterogeneity of tumour cells and genomic instability, these therapeutic interventions confer benefits to only a fraction of patients and grapple with challenges such as drug resistance and relapse.2 Hence, it is still urgent and difficult to explore the potential therapeutic targets of NSCLC and identify the underlying pathogenesis to achieve a better prognosis for NSCLC patients.
Tumour cells can change the metabolic patterns of key nutrients to favourably alter their direction, a characteristic metabolic shift referred to as metabolic reprogramming. Metabolic reprogramming has been recognized as a hallmark of tumours, mediating not only the unlimited proliferation of tumour cells but also bringing new hope for targeted therapies against tumours by addressing their abnormal metabolism.3 However, metabolic reprogramming makes it challenging to achieve desired therapeutic effects through direct targeting of metabolic mechanisms. It is imperative to further study the molecular mechanisms of abnormal tumour metabolism and consequently develop indirect targeting strategies for tumour metabolism.4
Glycolysis, a conserved metabolic pathway, involves the conversion of glucose into pyruvate, generating adenosine triphosphate (ATP) and intermediates for biosynthesis. In normal cells, energy production predominantly occurs through oxidative phosphorylation; however, cancer cells exhibit a heightened reliance on glycolysis, even in the presence of oxygen, a phenomenon known as the “Warburg effect”. This metabolic adaptation not only satisfies the increased energy demands of rapidly dividing cells but also provides metabolic intermediates crucial for biomass synthesis. Previous studies have demonstrated that a high aerobic glycolysis rate is related to tumour occurrence, progression and drug resistance.5, 6 Aerobic glycolysis is an aberrant metabolic pattern prevalent in many solid tumours, including NSCLC, and has proved to be a novel focal point for cancer therapy (Figure 1). During this process, the glycolysis rate is restricted by hexokinase, phosphofructokinase-1, pyruvate kinase lactate dehydrogenase and so on. Hexokinase 2 (HK2) is an essential kinase for glucose metabolism and is associated with tumour cell proliferation through enhanced aerobic glycolysis.7 Glycolytic inhibitor 2-DG, a small-molecule inhibitor of HK2, can inhibit ERK activation by activating the LKB1/AMPK signalling pathway, which can negatively regulate the IGF1R signalling pathway and RAS activity.8 Recent studies have identified key oncogenes and tumour suppressors that modulate glycolytic pathways. For instance, activation of oncogenes signalling pathways, such as PI3K/Akt, MYC and Gankyrin, enhances glucose uptake and promotes glycolysis. Gankyrin, which is upregulated in NSCLC, promotes glycolysis by promoting the expression of YAP1. Moreover, silencing Gankyrin was found to inhibit the expression of key glycolytic enzymes HK2, PKM2, PGK1 and LDHA at the transcriptional and protein levels, while enhancing lactate and ATP production.5 Tumour suppressors like p53, conversely, exert inhibitory effects on glycolysis, highlighting the intricate regulatory network governing metabolic reprogramming.9 Additionally, interactions between tumour cells and stromal components contribute to metabolic alterations, emphasizing the importance of studying glycolysis in the context of the tumour niche.10 Therefore, it is of great significance to understand the molecular mechanism of metabolic enzymes regulating aerobic glycolysis and mediating the tumorigenesis and development of NSCLC.
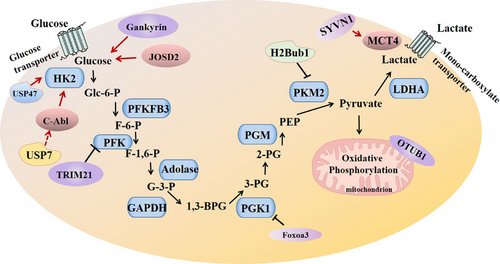
In this issue of Clinical and Translational Medicine, He et al.11 identified a novel regulatory cascade that may play a vital role in the progression of NSCLC cell glycolysis. In He et al.’s work, they have elucidated the complex molecular mechanisms underlying NSCLC tumorigenesis. The investigation meticulously explores the role of the Abelson tyrosine kinase (c-Abl), a frequently mutated protein in NSCLC, elucidating its stability and regulatory mechanisms. The abnormal overexpression of a substantial number of deubiquitinases in tumours is associated with poor prognosis for cancer patients. The authors validated that the USP7/c-Abl axis promoted NSCLC cell glycolysis by upregulating HK2 protein. They detected an increase in the concentration of lactate and pyruvate, the product of glycolysis. Overexpression of c-Abl increased the protein levels of HK2 and GLUT2 in the cytoplasm, thereby fostering cellular glycolysis. Conversely, specific sgRNA-mediated knockout of c-Abl led to reductions in HK2 and GLUT2 proteins. The authors also revealed that c-Abl increases protein levels of HK2 and GLUT2 through the post-translational modification pathway, but does not affect their RNA levels, as this effect is independent of the NLS domain. Notably, c-Abl upregulated HK2 protein by phosphorylation and deubiquitination, independent of phosphorylation AKT K290R. Furthermore, USP7 also increased HK2 in association with c-Abl. Knockdown of either USP7 or c-Abl resulted in the suppression of NSCLC cell glycolysis and a reduction in lactate production. Both USP7 and c-Abl increased the lactate concentration, the product of glycolysis. Thus, altered expression of HK2 and GLUT2 by c-Abl and USP7 directly interfered with glycolysis process thereby modulating metabolic reprogramming of NSCLC.
The activities of glycolytic enzymes can be regulated by specific post-translational modifications, such as ubiquitination, acetylation and SUMOylation, in response to oncogenic signals, thereby promoting aerobic glycolysis. Studies have shown that E3 ubiquitin ligase SYVN1 mediated polyubiquitylation facilitates the localization of MCT4, a critical lactate transporter, in the plasma membrane to promote the progression of lung adenocarcinoma.12 On the contrary, mitochondria-localized OTUB1 modulates aerobic glycolysis by inhibiting K48-linked ubiquitination and turnover of OXPHOS proteins.13 In He et al.’s work, they further investigated the molecular mechanisms underlying the regulation of NSCLC cell glycolysis by USP7 and c-Abl. By utilizing combined techniques and strategies, including the deubiquitinase library screen, the affinity-purification coupled tandem mass spectrometry, and Co-immunoprecipitation, pull-down, western blotting and a mice model experiment, they identified USP7 as a putative deubiquitinase of c-Abl. Specifically, USP7 interacts with c-Abl and prevents it from K48-linked polyubiquitination therefore stabilizing c-Abl and activating its kinase activities. Importantly, the study reports that various c-Abl mutants, associated with NSCLC, can be deubiquitinated and stabilized by USP7. Subsequently, the study further highlights the role of USP7 in promoting the accumulation of c-Abl in the cytoplasm by enhancing its binding with 14-3-3α/β, consequently activating the oncogenic c-Abl signalling pathway.
The excellent study by He et al. extends our understanding about the c-Abl deubiquitination modification in the field of NSCLC cell glycolysis. Targeting the USP7-c-Abl-HK2 axis represents a promising therapeutic strategy for hyperglycolytic NSCLC. Having elucidated the molecular mechanisms by which USP7 inhibits c-Abl ubiquitination and enhances c-Abl stability, the authors further demonstrated that the coordinated activities of USP7 and c-Abl promote NSCLC pathogenesis, including cell proliferation, migration, and tumour growth. Noteworthy is the observation that c-Abl is markedly overexpressed in NSCLC tissues, imparting resistance to anti-NSCLC drugs. c-Abl functions as an oncogene in NSCLC and promotes NSCLC cell proliferation and contributes to drug resistance. Treatment with cisplatin and doxorubicin induced apoptosis in NSCLC cells but the apoptosis levels were markedly reduced by overexpression of c-Abl. Validation of these findings was achieved through xenograft mouse models, where subcutaneous injection of USP7-deficient H1299 and USP7-overexpressing A549 cells underscored the pivotal roles of USP7 in mediating NSCLC cell growth. Furthermore, the study elucidated the regulatory impact of USP7 on c-Abl in the context of NSCLC. These insights not only deepen our understanding of the intricate interplay between USP7 and c-Abl in NSCLC pathogenesis but also bear significant implications for the development of targeted therapeutic strategies.
In conclusion, elucidating the intricate relationship between NSCLC and glycolysis is crucial for identifying innovative therapeutic avenues in cancer treatment. The elegant study by He et al. not only unveils a substantial advancement in our comprehension of the molecular intricacies associated with c-Abl and USP7 regulation in NSCLC but also identifies the USP7/c-Abl axis as a pivotal regulator, thereby disclosing a novel regulatory mechanism poised to serve as a potential target for precision therapy in NSCLC. The role of c-Abl in modulation of the activities of other glycolytic enzymes has not been investigated in this work. Additional research is required to determine whether the observed desirable effects of c-Abl are entirely derived from the USP7 deubiquitination modification. Despite these limitations, these findings underline the importance of continued research in unraveling the complexities of NSCLC glycolysis for the formulation of effective therapeutic strategies.
AUTHOR CONTRIBUTIONS
Drs. Yu, Jiang and Shan contributed to the preparation and collection of original literature and figures and the writing and editing of the manuscript.
ACKNOWLEDGEMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
Not applicable.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




