Short-term cyclosporin A treatment improves pregnancy outcomes in patients with unexplained pregnancy loss: A prospective, open-label, observational study
Abstract
Background
Various treatments to improve live birth rate in women suffering unexplained recurrent pregnancy loss (URPL) have proven ineffective. Cyclosporin A (CsA), an immunosuppressant widely used in organ transplantation, can induce maternal–foetal immune tolerance and improve trophoblast cell biological function. Whether CsA administration in the first trimester increases live birth rate in URPL women remains unknown.
Methods
A single-centre, prospective, observational cohort study of URPL patients treated at Obstetrics and Gynecology Hospital of Fudan University was conducted between 2010 and 2016. Participants within 6 weeks of gestation were divided into two groups: CsA or dydrogesterone. To compare efficacy and safety of interventions, data describing maternal and foetal conditions throughout 180 days after labour were collected. Mathematical models were used to identify the miscarriage risk factors in the subsequent pregnancy.
Findings
908 of 1931 women aged 18–40 with naturally conceived children and URPL history were recruited and received CsA (n = 660) or dydrogesterone (n = 248) treatment. The live birth rates in CsA and dydrogesterone groups were 88.31% and 89.36%, respectively (relative risk 0.99; 95% confidence interval 0.94–1.04; p = .72). Except for ectopic pregnancy only in dydrogesterone group (p = .005), there was no significant difference in outcomes of miscarriage, preterm birth, full-term birth, stillbirth or continued pregnancy. There was no discernible difference between CsA and dydrogesterone groups regarding maternal complications, congenital foetal abnormalities and growth. Potential risk factors for miscarriage in URPL patients included maternal age, number of prior pregnancies and miscarriages, the levels of serum progesterone, regulatory T-cell (Tregs, CD4+CD25+CD127−T), blocking antibody and anti-idiotypic antibody.
Interpretation
In women with URPL, short-term CsA therapy during the first trimester may increase the likelihood of live birth. The effectiveness of CsA is comparable to dydrogesterone without increasing risk of maternal complications or foetus congenital abnormalities. For women experienced URPL, CsA may be a potential therapeutic medicine.
Pregnancy loss (or miscarriage) is a prevalent and distressing complication of pregnancy.1, 2 Recurrent pregnancy loss (RPL), most occurred in the first trimester of pregnancy,3, 4 is identified in women who lose two or more clinically recognized and consecutive pregnancies.1 It affects ∼5% of couples (2.5% of women) who attempt to conceive.1, 2 Unexplained RPL (URPL) accounts for more than 50% of women who experience RPL.1, 4, 5 The causes of URPL are unknown, which may be associated with psychological and physical burdens or higher levels of proinflammatory Th1 cytokines and Th17 cells.5-7 The embryo can be viewed as an allogeneic graft expressing paternal antigen,3, 7, 8 and sufficient placental development is essential for successful pregnancy9; thus, maternal–foetal immune intolerance and trophoblast dysfunction have been postulated as important causes of URPL.3, 10 The pathogenesis of URPL has been found to be similar to that of organ rejection among solid-organ transplant recipients.11, 12
Therapy for URPL remains controversial because of its undefined cause. Although progesterone, such as dydrogesterone, has become a commonly used supplement or intervention drug in women with RPL or URPL, its efficacy is still debatable, and there are few specific interventions for these women.4, 5, 13, 14 Cyclosporin A (CsA) is a potent immunosuppressive agent that is widely administered to prevent alloreactivity and treat identified autoimmune diseases.3, 7 CsA mainly inhibits T-cell proliferation and activation and influences the functions of multiple immune cells, achieving immunosuppression.3, 7, 10, 15 Based on the assumption that unexplained miscarriage may be due to maternal–foetal immune intolerance and impaired trophoblast cell function, women with URPL may benefit from treatment with CsA.3, 10 Our earlier studies revealed that low-dose CsA supports the biological functions of trophoblast cells and maternal–foetal immunotolerance in addition to its powerful immunosuppression effect.3, 9, 16 We also demonstrated that administration of low-dose CsA induced maternal T-cell tolerance to paternal antigens at the window of implantation in abortion-prone mating mice, skewing towards a Th2 bias at the maternal–foetal interface.3 Low-concentration CsA treatment enhanced the proliferation and invasion of human first-trimester trophoblasts in abortion-prone mating mice, contributing to successful pregnancy.3, 10, 17 Several signalling pathways may play roles in how CsA promotes the invasion and migration of human trophoblast cells.13, 14, 17
Regarding pregnancy safety, the US Food and Drug Administration classifies CsA as a class C drug. Animal reproduction studies indicate that only higher dosages of CsA (2–10 mg/kg/d) may negatively affect the foetus.20, 21 The safety of taking CsA during pregnancy has been confirmed in several studies.20, 22
Recently, the potential for employing CsA in women with URPL or RPL was investigated in terms of its efficacy and safety during pregnancy. It was reported that CsA administration reduced the Th1/Th2 ratio and enhanced pregnancy outcomes.7 Low-dose CsA increases the live birth rate of patients with URPL who underwent in vitro fertilization and embryo transfer with no maternal–foetal adverse events, which may be related to the immune regulation of CsA. Moreover, the National Intellectual Property Administration in China has also approved the use of low-dose CsA to increase the live birth rate in patients with URPL (patent number ZL03108566.0). However, only limited sample sizes or retrospective analyses have been used to study the use of CsA in individuals with URPL. Our study examined the efficacy and safety of CsA compared with that of dydrogesterone (6-dehydro-9β,10α-progesterone) in treating URPL in women, and the results may supply evidence for the application of CsA in miscarriage-prone women.
1 MATERIALS AND METHODS
1.1 Study population
Patients were enrolled between January 2010 and December 2016 at the Fudan University Obstetrics and Gynecology Hospital (Shanghai, China). The enrolled participants met the entry criteria of 18–40 years of age, natural conception with a gestational age less than 6 weeks and a history of URPL, defined as two or more consecutive pregnancy losses before 24 weeks of gestation.1 Patients with clear-cut causes of pregnancy loss, such as an abnormal parental karyotype, uterine anatomic structural abnormalities (assessed by ultrasonography, hysterosonography or hysterosalpingogram), chronic infections, a pathogen infection (assessed by TORCH inspection), autoimmune diseases confirmed by a rheumatologist, hormonal and metabolic disorders and thrombotic disease, were excluded; all other participants were diagnosed with URPL. Additionally, we disqualified those with a history of hypertension, heart disease or mental illness and those who could not finish the clinical trial within a year or who refused to participate in monotherapy. Before participating in the trial, every eligible participant signed written informed consent forms. The present study was approved by the Ethics Committees of the Obstetrics and Gynecology Hospital, Fudan University, and was registered at www.chictr.org.cn as a single-centre, open-label trial (ChiCTR-TRC-14004216).
1.2 Exposure categories and drug regimens
CsA or dydrogesterone was administered to participants whose gestational ages were fewer than 6 weeks, depending on the doctor's judgment and the patient's preferences. There were no other clinical signs for the prophylactic therapy of spontaneous abortion aside from a history of URPL. Clinical practitioners created the treatment plan based on their personal experience and basic research results.
According to the therapy they received, patients were split into two groups: the CsA group and dydrogesterone group (control group). As soon as the participants obtained a positive result on a pregnancy test, but not later than 6 weeks of gestation, patients in the CsA group were administered a dosage of 50 mg of CsA every 8 h, whereas patients in the dydrogesterone group received 10 mg of dydrogesterone orally every 8 h. Patients received CsA for at least 20 days but no longer than 30 days. Both medications were withdrawn when the serum human chorionic gonadotropin (HCG) level was higher than 100 000 mIU/ml or the embryo or when an ultrasound showed that the embryo or foetus was beating. For patients in the CsA group, the CsA concentration needed to be determined, including the C0 (concentration prior to medication) and C2 (concentration after 2 h of medication) concentrations. If the C0 concentration was less than 40 ng/ml or the C2 concentration was higher than 400 ng/ml, the CsA dosage was adjusted under transplantation experiences.24-26
1.3 End points and assessment protocol
The live birth rate at 22 full weeks of gestation served as the primary end point of the drug's effectiveness. A live birth was the delivery of a single newborn, a twin delivery was the delivery of two newborns27 and the live birth rate was the proportion of live births in each group with pregnancy outcomes.
The secondary end points included miscarriage (after 22 completed weeks of gestation), preterm birth (due to concerns regarding a possible increased risk of preterm delivery with the use of CsA during pregnancy20), full-term birth, stillbirth, ectopic pregnancy, continued pregnancy outcomes beyond 12 weeks, the gestational age of the newborns at birth and embryo chromosomal abnormalities in women who had miscarriages. For a thorough assessment of effectiveness and safety, neonatal characteristics, including birth sex, height and weight, were also recorded, and the survival rate of neonates at 42 and 180 days was calculated. Obstetrical complications throughout pregnancy and at 42 and 180 days after birth, as well as neonatal events on the day of delivery and at 42 and 180 days of follow-up, were also used to assess safety results. Obstetrical complications included hypertension during pregnancy or preeclampsia (defined as de novo hypertension present after 20 weeks of gestation combined with proteinuria [>300 mg/day], other maternal organ dysfunction, such as renal insufficiency, liver involvement, neurological or haematological complications, and uteroplacental dysfunction, or foetal growth restriction), gestational diabetes mellitus (defined as a fasting glucose level higher than 7.0 mmol/L or a 2-h postload glucose level higher than 7.8 mmol/L according to the World Health Organization guidelines published in 2013),28 infectious diseases and carcinoma. Neonatal measurements, including height, weight, aural comprehension, congenital and developmental anomalies and the incidence of infectious diseases and carcinoma, were recorded on the day of delivery and at 42 and 180 days of follow-up.
We investigated biochemical and immunological indices during pregnancy and baseline characteristics to determine the predictive risk factors for miscarriage. HCG levels, progesterone levels, peripheral CD3+, CD4+, CD25+ and CD127− T cells percentages, CD3− blocking efficiency (BE), CD4− BE, CD25− BE, blocking antibody levels and anti-idiotypic antibody levels were all measured pre- and posttreatment (when the patients stopped using CsA or dydrogesterone).
Participants were involved in the study until 180 days after delivery or pregnancy loss. A trained research assistant collected the patients’ demographic information and medical histories at the visits, extracting clinical information about the therapy strategy, biochemical indices during pregnancy, delivery and foetal outcomes at birth. Telephone interviews were also conducted to acquire information on maternal and infant health after the mother and child were discharged from the hospital.
1.4 Statistical analysis
All clinical data were collected, and the results between CsA group and dydrogesterone group were compared. Categorical variables are expressed as the number, median (range, min and max) or frequency (%). Continuous variables are summarized as the means with standard deviations (SDs) or medians with interquartile ranges for normal and skewed distributions. Two-sample t tests (α = .05) and chi-square tests (or Fisher's exact test when appropriate) were used to analyse the differences in outcomes between two groups. The relative risk (RR) and 95% confidence interval (CI) were calculated by Cox proportional hazard regression analysis (risk ratio function).
Correlation analysis (for data visualization and multicollinearity testing via the ggplot function and variance inflation factor [VIF] calculation separately) was performed before performing logistic regression analysis to identify variables associated with miscarriage. Potential confounders, including maternal age (<20, 20–29, 30–39, and ≥40 years of age), body mass index (<30 and ≥30.0 kg/m2), the number of previous miscarriages (2, 3, ≥3), parity (0, 1, and ≥1), smoking status (a smoker was defined as a patient who had smoked ≥1 cigarette/day) and other related covariates, were retained in the models according to previous literature, regardless of statistical significance. Analysis in this study was two-tailed (α = .05), and a p < .05 was considered statistically significant. The RStudio software (RStudio, Inc., Cary, North Carolina) was used to analyse the data, and GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA) was used to generate all figures.
2 RESULTS
2.1 Study population and baseline characteristics
A total of 1931 women with URPL history who had naturally conceived pregnancy with a gestational age less than 6 weeks, and had treatment at the Fudan University Obstetrics and Gynecology Hospital, were evaluated from January 2010 to December 2016. A total of 908 women were eligible for the study based on our requirements and agreed to receive one medication; these women signed informed consent forms before participating in the trial. Participants were divided into two groups according to the therapy they received depending on the doctor's judgement and their own willingness: the CsA (n = 660) and dydrogesterone (control group, n = 248) groups. Overall, 4.4% of cases were lost to follow-up. A total of 868 of the 908 participants completed the study (633 and 235 participants in the CsA and dydrogesterone groups, respectively) (Figure 1). The baseline characteristics are shown in Table 1, and there was no significant difference between the two groups.
| Characteristics | Cyclosporine A (n = 660) | Dydrogesterone (n = 248) | p-Value |
|---|---|---|---|
| Basic characteristics | |||
| Age | 30.05 ± 3.42 | 30.29 ± 3.72 | .36 |
| BMI | 21.41 ± 2.53 | 21.59 ± 2.90 | .41 |
| BMI ≥ 30 | 2/574 (0.35%) | 3/226 (1.3%) | .14 |
| Blood pressure (normal) | 654/657 (99.54%) | 242/243 (99.59%) | 1 |
| Gestation characteristics | |||
| Number of preterm births | 0.01 ± 0.11 | 0.004 ± 0.06 | .39 |
| Number of miscarriages | 2.48 ± 0.76 | 2.52 ± 0.77 | .43 |
| First miscarriage (w) | 7.36 ± 2.71 | 7.51 ± 2.65 | .47 |
| Second miscarriage (w) | 7.37 ± 2.48 | 7.47 ± 2.46 | .57 |
| Third miscarriage (w/n) | 7.57 ± 2.95/251 | 7.25 ± 3.01/104 | .36 |
| Interquartile range (min, max) | (1, 25) | (1, 27) | |
| Fourth miscarriage (w/n) | 7.51 ± 3.75/63 | 8.77 ± 5.98/22 | .35 |
| Interquartile range (min, max) | (1, 22) | (4, 28) | |
| Smoking | |||
| None | 660/660 (100%) | 247/248 (99.60%) | .27 |
| ≤10 cigarettes/day | 0/660 (0%) | 1/248 (0.40%) | .27 |
| Alcohol use | 0/660 (0%) | 0/248 (0%) | 1 |
| Pet owners | 10/660 (1.52%) | 3/248 (1.21%) | 1 |
| Disease history | |||
| Liver disease | 5/660 (0.76%) | 6/248 (2.42%) | .08 |
| Kidney disease | 4/660 (0.61%) | 0/248 (0%) | .58 |
| Antiphospholipid antibody positive | 4/577 (0.69%) | 1/218 (0.46%) | 1 |
| Uterine cavity | |||
| Normal | 352/557(53.33%) | 126/214(58.88%) | .28 |
| Abnormal | 110/557(16.67%) | 38/214(17.76%) | .61 |
| Normal after treatment | 95/557(14.39%) | 50/214(23.36%) | .05 (.05053) |
| Oviducts | |||
| Normal | 318/560 (56.79%) | 109/212(51.52%) | .19 |
| Abnormal | 235/560 (41.96%) | 100/212(47.17%) | .19 |
| Normal after treatment | 7/560 (1.25%) | 3/212(1.42%) | 1 |
| Laboratory examination (before treatment) | |||
| Liver (normal) | 538/539(99.81%) | 185/185(100%) | 1 |
| Kidney (normal) | 542/542(100%) | 186/187(99.47%) | .26 |
| Reproductive hormones | |||
| LH | 5.44 ± 4.35 | 5.33 ± 2.83 | .74 |
| FSH | 7.10 ± 2.68 | 7.50 ± 5.60 | .45 |
| E2 | 70.66 ± 67.39 | 66.14 ± 78.79 | .57 |
| RPL | 55.52 ± 113.30 | 46.75 ± 113.98 | .46 |
| T | 5.76 ± 15.47 | 6.90 ± 19.40 | .56 |
| P | 1.32 ± 3.09 | 1.43 ± 2.59 | .81 |
| Pregnancy method | |||
| Natural pregnancy | 634/660(96.06%) | 243/248(97.98%) | .22 |
| In vitro fertilization | 26/660(3.94%) | 5/248(2.02%) | .22 |
- Abbreviations: BMI, body mass index; RPL, recurrent pregnancy loss.
2.2 Pregnancy outcomes
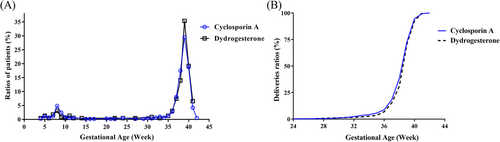
There was no significant difference in the two groups’ live birth rates, which were 88.31% (559/633) in the CsA group and 89.36% (209/235) in the dydrogesterone group (RR 0.99; 95% CI 0.94–1.04; p = .72). Additionally, there were no significant differences between the groups regarding miscarriage, preterm birth, full-term birth, stillbirth and continuing pregnancies beyond 12 weeks (Table 2). Ectopic pregnancy was only observed in the dydrogesterone group and was considerably more common than in the CsA group (p = .005). The miscarriage rates decreased but did not differ significantly after the participants with embryo chromosomal abnormalities were excluded (87 and 23 in the CsA and dydrogesterone groups, respectively). Participants in the CsA and dydrogesterone groups delivered newborns after an average of 38.49 ± 1.93 (Q1 = 38, Q3 = 40) and 38.76 ± 1.53 (Q1 = 38, Q3 = 40) weeks, respectively, with no significant difference (p = .05025). The distributions of the gestational age of the neonates at birth were similar and are shown in Figure 2. The delivery methods in the two groups were also similar and are shown in Table S1. In addition, neonatal characteristics, including birth sex, height and weight, were examined, and showed no differences between the two groups. In particular, the survival rates of the neonates at 42 and 180 days were 100% and 100% for the CsA group and 98.77% and 99.4% for the dydrogesterone group (RR 1.01; 95% CI 1–1.03; p = .07; RR 1.01; 95% CI 1–1.02; p = .25).
| Cyclosporin A (n = 633) | Dydrogesterone (n = 235) | Relative risk (95% CI) | p-Value | |
|---|---|---|---|---|
| Pregnancy | ||||
| Miscarriage (n, %) | 99/633 (15.64%) | 27/235 (11.49%) | 1.36 (0.93–2.15) | .13 |
| Miscarriage after removal of abnormal embryo data (n, %) | 87/633 (13.74%) | 23/235 (9.79%) | 1.40 (0.95–2.31) | .14 |
| Preterm birth (n, %) | 52/633 (8.21%) | 14/235 (5.96%) | 1.38 (0.82–2.69) | .31 |
| Term birth (n, %) | 481/633 (75.99%) | 189/235 (80.43%) | 0.94 (0.88–1.02) | .17 |
| Stillbirth (n, %) | 1/633 (0.16%) | 1/235 (0.43%) | 0.19 (0.01–2.97) | .47 |
| Ectopic pregnancy (n, %) | 0/633 (0%) | 4/235 (1.70%) | – | .005** |
| Live birth (n, %) | 559/633 (88.31%) | 210/235 (89.36%) | 0.99 (0.94–1.04) | .72 |
| Ongoing pregnancy at 12 weeks (n, %) | 541/620 (87.26%) | 206/229 (89.96%) | 0.97 (0.92–1.02) | .34 |
| Live births | ||||
| Gestational age at birth (weeks) (mean ± SD) (Q1, Q3) | 38.49 ± 1.93 (Q1 = 38, Q3 = 40) | 38.76 ± 1.53 (Q1 = 38, Q3 = 40) | .05 (.05025) | |
| Live birth before 28 weeks | 2/531 (0.38%) | 0/201 (0%) | 1 | |
| Live birth before 34 weeks | 17/531 (3.20%) | 3/201 (1.49%) | 1.62 (0.48–5.46) | .31 |
| Live birth before 37 weeks | 46/531 (8.66%) | 13/201 (6.47%) | 1.25 (0.69–2.26) | .37 |
| Twin live births (n, %) | 26/633 (4.11%) | 7/235 (2.98%) | 1.21 (0.53–2.75) | .55 |
| Birth weight (kg) | 3.32 ± 1.49 (Q1 = 3, Q3 = 3.6) | 3.46 ± 2.34 (Q1 = 3, Q3 = 3.6) | .42 | |
| Birth height (cm) | 49.53 ± 3.09 (Q1 = 50, Q3 = 50) | 49.75 ± 2.19 (Q1 = 50, Q3 = 50) | .27 | |
| Sex (male, [n, %]) | 265/554 (47.83%) | 98/211 (46.45%) | 1.03 (0.88–1.23) | .75 |
| Newborn survival to 42 days (n, %) | 472/472 (100.00%) | 161/163 (98.77%) | 1.01 (1–1.03) | .07 |
| Newborn survival to 180 days (n, %) | 488/488 (100.00%) | 165/166 (99.40%) | 1.01 (1–1.02) | .25 |
- Abbreviation: CI, confidence interval.
- ** p < .001.
The risk of maternal complications or foetal congenital anomalies was not increased by CsA treatment. Pregnancy-related obstetric complications included hypertension, preeclampsia, eclampsia, infection, gestational diabetes mellitus and post-partum haemorrhage (Table S2). Overall, 10.79% patients in the CsA group and 11.06% patients in the dydrogesterone group experienced anomalous maternal complications (RR 0.98; 95% CI 0.63–1.64; p = .89) (Table 3). Although the types and total number of foetal congenital anomalies in the CsA group (3.83%) were greater than those in the dydrogesterone group (2.45%), there were no significant difference between the two groups (RR 1.56; 95% CI 0.70–7.82; p = .50). Congenital heart disease accounted for the largest proportion of congenital anomalies in both groups. At 42 and 180 days of follow-up, neither group's maternal participants were afflicted with tumours, and there was no difference in any complications or infectious diseases (RR 0.19; 95% CI 0.01–3.08; p = .48). There were no obvious differences between the two groups of neonates in aural comprehension, developmental abnormalities, carcinomas or the probability of infection during follow-up, and both groups of neonates had similar physiques (heights and weights).
| Cyclosporin A (n = 633) | Dydrogesterone (n = 235) | Relative risk (95% CI) | p-Value | |
|---|---|---|---|---|
| Maternal outcomes | ||||
| Complications during pregnancy (n, %) | 56/519 (10.79%) | 22/199 (11.06%) | 0.98 (0.63–1.64) | .89 |
| Complications among 42 days (n, %) | ||||
| None | 489/491 (99.59%) | 191/192 (99.48%) | 1.00 (0.99–1.01) | 1 |
| Hypertension | 1/491 (0.20%) | 1/192 (0.52%) | 0.20 (0.01–3.13) | .48 |
| Others | 1/491 (0.20%) | 0/192 (0%) | – | 1 |
| Tumour at 42 days (n, %) | 0/490 (0%) | 0/191 (0%) | – | – |
| Infectious disease at 180 days (n, %) | 1/488 (0.20%) | 1/188 (0.53%) | 0.19 (0.01–3.08) | .48 |
| Tumour at 180 days (n, %) | 0/489 (100%) | 0/189 (100%) | – | – |
| Neonatal outcomes | ||||
| Neonatal congenital anomalies at birth (n, %) | 21/548 (3.83%) | 5/204 (2.45%) | 1.56 (0.70–7.82) | .50 |
| Jaundice at 42 days (n, %) | 167/517 (32.43%) | 48/192 (25.00%) | 1.29 (0.99–1.72) | .07 |
| Abnormal dysplasia at 42 days (n, %) | 9/427 (2.11%) | 0/172 (0%) | – | .07 |
| Abnormal hearing at 42 days (n, %) | 3/409 (0.73%) | 1/175 (0.57%) | 0.65 (0.07–6.16) | 1 |
| Weight after 180 days (kg) (mean ± SD) (Q1, Q3) | 8.58 ± 4.47 (Q1 = 7.8, Q3 = 9) | 8.62 ± 4.60 (Q1 = 7.5, Q3 = 9) | – | .91 |
| Height after 180 days (cm) (mean ± SD) (Q1, Q3) | 68.20 ± 5.40 (Q1 = 67, Q3 = 70) | 68.14 ± 5.06 (Q1 = 67, Q3 = 70) | – | .88 |
| Abnormal dysplasia at 180 days (n, %) | 15/483 (3.11%) | 3/184 (1.63%) | 1.44 (0.42–4.90) | .42 |
| Tumour at 180 days (n, %) | 0/479 (0%) | 0/184 (0%) | – | – |
| Infectious disease at 180 days (n, %) | 2/476 (0.42%) | 2/182 (1.10%) | 0.26 (0.04–1.81) | .31 |
- Abbreviations: CI, confidence interval; SD, standard deviation.
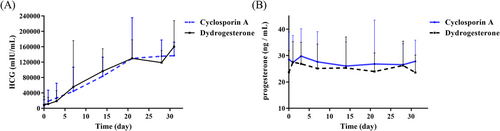
The biochemical indices of the two groups during pregnancy (including the serum HCG and progesterone levels) showed similar trends in the pretreatment period, 1, 3 and 7 days after treatment, 2, 3 and 4 weeks after treatment and 3 days after drug withdrawal. However, the HCG level showed a declined trend after treatment for 20 days in the dydrogesterone group (Figure 3). CsA concentrations during treatment were all within the acceptable limit (Table S4).
2.3 Predictive risk factors for miscarriage
| Estimated value | Standard error | Z-Value | p-Value | |
|---|---|---|---|---|
| (Intercept) | 4.67 | 0.79 | 5.93 | 3.08e−09*** |
| Age | −0.07 | 0.025 | −2.78 | .00542** |
| Number of abortions | −0.78 | 0.19 | −4.04 | 5.40e−05*** |
| Progesterone (P) | 0.07 | 0.08 | 0.93 | .35246 |
| Number of gestations | 0.48 | 0.18 | 2.68 | .00733** |
| CD25+ T cells | −0.05 | 0.003 | −1.41 | .15838 |
| CD127− T cells | 0.014 | 0.008 | −1.76 | .07819 |
| Blocking antibody | 0.01 | 0.005 | −1.83 | .06758 |
| Anti-idiotypic antibody | 0.007 | 0.005 | 1.62 | .10620 |
- *** p < .0001.
- ** p < .001.
| Variable coefficient | |
|---|---|
| (Intercept) | 106.70 |
| Age | .93 |
| Number of abortions | .46 |
| Progesterone (P) | 1.07 |
| Number of gestations | 1.62 |
| CD25+ T cells | .95 |
| CD127− T cells | 1.01 |
| Blocking antibody | 1.01 |
| Anti-idiotypic antibody | 1.007 |
- Note: For example, the probability of successful foetal protection is 0.93 times that of failure when the age increases by 1 year.
3 DISCUSSION
This cohort study demonstrated that CsA or dydrogesterone monotherapy could help pregnant women suffering URPL have a wonderful pregnancy outcome. The miscarriage rates in women who received CsA or dydrogesterone monotherapy were low. In addition, the live birth rates following CsA or dydrogesterone monotherapy were similar in our study. This cohort is the largest and most time-consuming study on the efficacy of and safety of CsA administration during first-trimester pregnancy on the pregnancy outcomes of patients suffering URPL.
There are few definitive studies on the safety of CsA during pregnancy. CsA is usually administered in pregnancy with other diseases such as transplantation and autoimmune diseases, and pregnancy outcomes were usually evaluated based on the drug combination and primary disease in previous investigations.22, 29 According to the European League Against Rheumatism, current evidence based on single drug exposure reports has revealed that the lowest effective dose of CsA would not increase the rate of congenital malformations,29 which is why our trial assessed its effectiveness as a monotherapy. The preterm, term and stillbirth rates of the CsA monotherapy group were comparable to those in the dydrogesterone monotherapy group in our study, but ectopic pregnancies occurred only in the dydrogesterone group, consistent with the results of Coomarasamy et al.14
Additionally, we observed that neither the risk of maternal complications (hypertension, preeclampsia and gestational diabetes) nor the adverse effects associated with the potent immunosuppression (such as infections or carcinoma) increased in the CsA group when compared to the dydrogesterone group. The overall incidence of foetal congenital anomalies in the CsA group was lower than that of the general population or of patients with organ transplantation and autoimmune disease.30 The newborns in both groups also exhibited normal heights and weights, providing that CsA treatment during pregnancy did not affect the growth and development of newborns. This finding is consistent with the results of the follow-up studies for the offspring of mothers who had autoimmune diseases.31 However, a meta-analysis revealed that using CsA might be linked to an increase in preterm. Due to the lack of available evidence, confirming the long-term safety of CsA use after pregnancy is challenging. It is necessary to investigate additional long-term adverse consequences of CsA on pregnant women's health and infants’ physical and mental development.30
This was the first report describing the levels of HCG and serum progesterone in patients with URPL during drug therapy. Strangely, 29.41% (5/17) of the participants in the dydrogesterone group had aberrant HCG values at 4 weeks after the start of medication. The serum progesterone levels in the dydrogesterone group decreased immediately when the medicine was stopped, whereas those in the CsA group gradually increased. In both groups, the average serum progesterone concentration was similar to the mean serum progesterone concentration on the day of embryo transfer in patients with ongoing pregnancy,32 which may be due to the scant monitoring information for HCG and serum progesterone levels collected in our study. The potential function of CsA in serum progesterone levels could be explored in the future.
The risk factors for miscarriage were also investigated by mathematical models in our study. It was demonstrated that potential risk factors for miscarriage in patients with URPL included age, the number of miscarriages, serum progesterone level, the number of gestations, Treg-(CD25+CD127−) cell proportion, blocking antibody level and anti-idiotypic antibody level. Maternal or parental age significantly impacted the alleged cause of the miscarriage.5, 33 The highest risk of miscarriage was detected at 45 years of age and above (53.6%) due to chromosomal aberrations increasing with age.5, 14 The next highest rate was 15.8% in the youngest mothers aged under 20 years,5 for whom the level of sperm DNA integrity might be the main influencing factor of paternal age on miscarriage.33 The number of previous miscarriages had a detrimental impact on the outcome of the subsequent pregnancy, particularly for those who had experienced six or more prior losses, for whom the miscarriage rate reached 53%34. Additionally, it was demonstrated that pregnancy history (preterm delivery, stillbirth and caesarean section) and pregnancy complications (gestational diabetes) somewhat increased the risk of miscarriage,5 and the serum progesterone level, as a significant prognostic factor for pregnancy outcomes and the live birth rate, was substantially connected with reduced pregnancy rates when the level was below 10 ng/ml,32, 35 which is also consistent with our data. Numerous T cells and regulatory T cells (Tregs) assist in mediating implantation success and establishing pregnancy tolerance and are linked to an imbalance in unexplained infertility, miscarriage or preeclampsia.36 In our study, CD25+ T cells and CD127− T cells, which are the main targets of CsA, were identified as potential miscarriage-preventive factors for miscarriage,37 which are a potential mechanism of CsA in the treatment of URPL. Additionally, blocking antibodies and anti-idiotypic antibodies contribute significantly to protecting the foetus by preventing matrix cytotoxic T cells and covering paternal human leukocyte antigen on the foetus's surface.37 However, it is still debatable whether blocking antibodies have any prognostic or predictive utility for URPL.24 The association between risk factors and miscarriage rate predicted by mathematical models should be confirmed by further clinical observations.
To our knowledge, this is the first study to examine the therapeutic efficacy of CsA in women with a history of URPL and to compare the development of the foetus to that of dydrogesterone. It also has a large sample size and close follow-up. There are some limitations to the current study. First, a randomized controlled trial will be necessary to confirm our findings in the future because, in our open-label study, the number of participants willing to be enrolled in the CsA group was significantly higher than that in the dydrogesterone group due to their fear of miscarriage again if choosing dydrogesterone. Second, the sample size of the current study was not large enough, and the long-term maternal and foetal side effects of CsA need further evaluation. The enrolled patients and neonates are still being monitored, and long-term safety data on CsA or dydrogesterone for RPL will be reported in our subsequent studies. Third, even though we utilized mathematical models to anticipate the risk factors, it was difficult to assess the real mechanism of medication intervention in our study because immunological examination data, such as blocking antibody levels, were not collected after CsA or dydrogesterone treatment. Fourth, the CsA group was compared with the dydrogesterone group instead of a control group. There are two very important reasons. On the one hand, getting ethical permission for clinical regimens that use placebo therapy on RPL patients can be challenging. On the other hand, patients with RPL are constantly anxious and wish to conceive but are undoubtedly unwilling to receive either no treatment or a placebo. Finally, supportive care was helpful in improving URPL in a dedicated clinic,34 and combined medication with psychotherapy for patients with URPL is also worth exploring in the future.
4 CONCLUSION
Like dydrogesterone, our results demonstrated that CsA, a popular and powerful immunosuppressive medicine, may effectively increase the likelihood of live birth in women with a history of URPL. CsA monotherapy did not increase the risk of maternal complications or foetal congenital anomalies and did not hamper the neonatal growth and development. CsA might be a helpful agent to lower the chance of miscarriage for women with URPL, but larger, randomized, controlled trials are required to support this result.
ACKNOWLEDGEMENTS
The obstetricians and gynaecologists who dedicated their professional time and assistance to this study and all the women who took part in it have our sincere gratitude. This research was funded by funds from the National Natural Science Foundation of China and the Ministry of Science and Technology of China (2017YFC1001400 and 2015CB943300 to Da-Jin Li, respectively) (32070915 to Da-Jin Li).
CONFLICTS OF INTEREST
No conflicts of interest exist, according to the authors, with the publishing of this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




