Botulinum toxin therapy for isolated first dorsal interosseous muscle pain and hypertrophy: A case report
Key Clinical Message
Botulinum toxin (BTX) injection can be an effective treatment for persistent pain and functional impairment associated with hypertrophy of the first dorsal interosseous muscle. It offers a non-surgical and minimally invasive alternative for those who have failed conservative treatment, showcasing the therapeutic promise of BTX for addressing similar musculoskeletal conditions.
1 INTRODUCTION
Skeletal muscle hypertrophy can result from various factors, including congenital conditions, strength training, overuse, neuromuscular disorders, compensatory responses, or systemic diseases.1-5 However, isolated hypertrophy of a particular muscle, such as the first dorsal interosseous (FDI) muscle, is unusual.6-9
The FDI muscle plays a crucial role in hand function, particularly in tasks that require grip and pinch strength. Hypertrophy and associated pain can lead to functional limitations and disability, affecting a person's quality of life.10, 11
Botulinum toxin (BTX) is a highly potent neurotoxin that has proven effective in managing various musculoskeletal disorders. Its applications in conditions such as muscle spasticity, dystonia, and myofascial pain syndrome have shown promising results. The unique capacity to target and inhibit specific muscle contractions has become an invaluable asset in the medical field, offering patients relief and enhancing their overall quality of life.12, 13
We describe a case of a woman with unilateral FDI muscle pain and hypertrophy, who experienced substantial relief following a BTX injection.
2 CASE HISTORY/EXAMINATION
A 38-year-old right-handed woman was referred to the physical medicine and rehabilitation clinic with a 10-month history of progressive pain and swelling in her right first dorsal web space. She described the pain as constant, and repetitive tasks involving writing exacerbated her symptoms. The patient noted that the pain worsened during hand activities and improved with rest, but it did not completely resolve even during periods of extended rest. The Numeric Rating Scale (NRS) was 10 during repetitive hand use, such as writing, and was 5 at rest. She did not complain of sensory changes or any other accompanying symptoms. She had no specific occupation, engaging exercises, or played any instruments. The patient's medical history was unremarkable, with no history of trauma.
On physical examination, a visible enlargement was noted in the right first dorsal web space. No erythema, ecchymosis, or skin changes were observed. A firm, tender, and non-mobile mass was palpated without any warmth or fluctuance. Hand movements were limited by pain, especially during gripping, and grip strength was reduced on the right side compared to the left due to pain. Sensation was intact to light touch and pinprick throughout the hand and fingers. There were no signs of neurological deficits or other systemic involvement.
3 METHODS
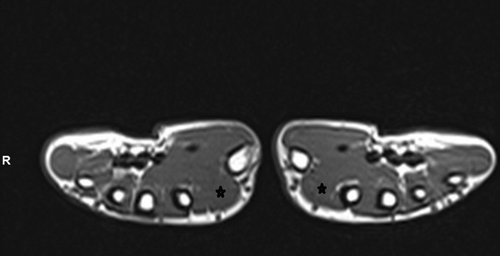
Differential diagnoses included a soft tissue tumor, muscle or tendon sheath tumor, ganglion cyst, localized myositis, muscle strain, and neurogenic hypertrophy. The patient underwent thorough investigations to evaluate the mass, ruling out potential issues. Laboratory tests were within normal limits. The hand x-ray displayed no abnormalities, while ultrasonography revealed an enlargement in the FDI muscle. MRI showed mild hypertrophy of the FDI muscle with preserved architecture and regular signal intensity (Figure 1). No evidence of a tumor or any abnormal structure or vasculature was observed. Nerve conduction study (NCS) results were within normal limits, and electromyography (EMG) demonstrated normal motor unit recruitment and morphology without any evidence of denervation.
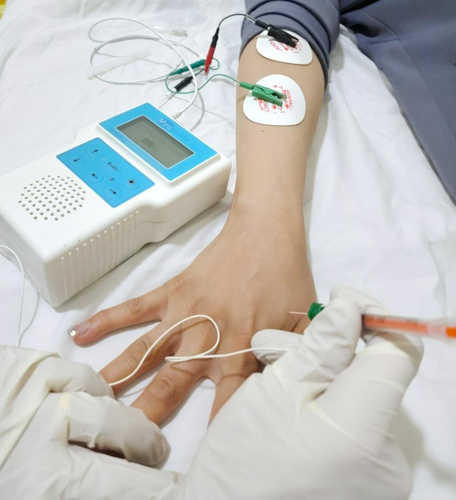
The patient had multiple appointments, which were managed conservatively with nonsteroidal anti-inflammatory drugs, physical therapy, acupuncture, and local corticosteroid injection. However, these interventions provided only minimal relief. After discussing potential treatment options, the patient consented to receive a BTX injection. Under sterile conditions, the FDI muscle was identified using EMG guidance (Figure 2). The faculty of physical medicine and rehabilitation injected a total of 10 units of BTX (Dysport 300 IU) using a 30-gauge needle into one injection site in the hypertrophied muscle. Post-injection, the patient was advised to avoid strenuous activities.
4 RESULTS
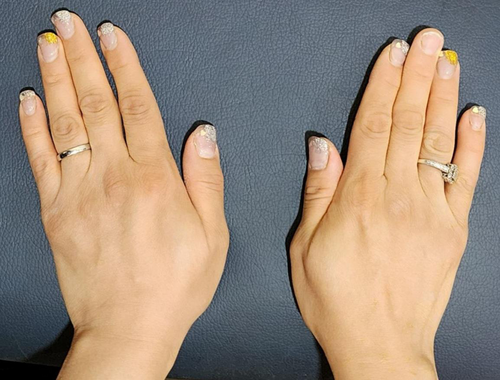
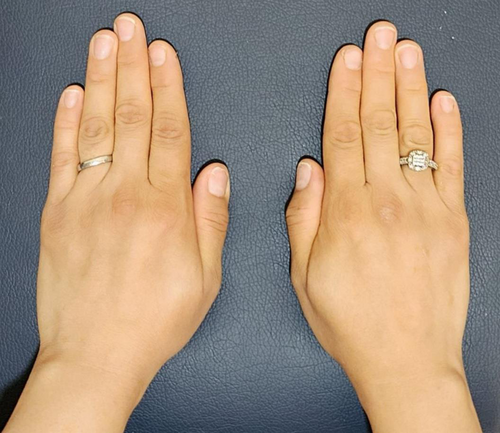
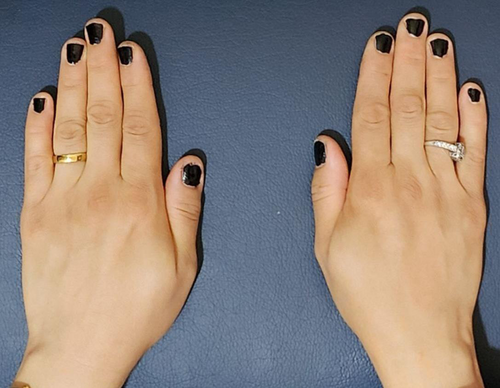
The patient reported a significant reduction in pain within 1 week following the BTX injection. She experienced pain improvement (NRS: 1-2) and was able to perform activities of daily living without discomfort. Regular follow-up visits were scheduled to assess progress at 1 month, 3 months, and 6 months. The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire,14 a validated questionnaire assessing upper extremity function, was used to monitor the patient's progress during follow-up visits. The initial DASH score was 70, indicating significant impairment. At the first follow-up (4 weeks post-injection), the DASH score improved to 35. At subsequent follow-up visits (12 weeks post-injection), the DASH scores further improve to 20, and at the last follow-up visit at 24 weeks, the DASH score became 23.3. Physical examination at the 3-month and 6-month follow-ups revealed a marked reduction in the size of the mass (Figures 3-6). The patient reported sustained pain relief and regained full functionality of her hand. No adverse effects were reported.

5 DISCUSSION
This case describes a patient with idiopathic hypertrophy of the FDI muscle, a rare condition characterized by asymmetric muscle enlargement without an identifiable cause. This condition can often be challenging to diagnose, as it presents similarly to other muscle abnormalities, such as tumors or pseudotumors. However, MRI imaging plays a crucial role in distinguishing between these conditions, as idiopathic hypertrophy typically does not display abnormal signal intensity or enhancing mass-like lesions. In the presented case, the patient's main complaints were pain and enlargement in the first dorsal web without any apparent causes, risk factors, or associated symptoms. MRI findings revealed enlargement of the FDI muscle on the affected side, with no presence of tumors, abnormal signal intensity, or accessory muscle. Additionally, EMG study results showed a normal pattern.
In general, muscle hypertrophy represents the normal physiological response to increased workload or mechanical stress. However, compensatory mechanisms following tendon or muscle tears, or inflammation around the tissue, can also cause muscle hypertrophy.1, 2, 15, 16
In this case, the patient's hypertrophy was not caused by physical activity or exercise, which usually impacts muscle groups rather than a single muscle. There was no history of trauma, and no signs of inflammation were detected through physical examination, lab tests, or MRI. The sole finding was the enlargement of an isolated muscle.
Non-tumoral muscle conditions that mimic tumors or pseudotumors include muscle injuries (strains, overuse, hematoma, and contusions), inflammation or infection, muscle herniation, and unusual hand muscles (accessory, hypertrophic, or abnormal extensions of proximal muscles).8, 15-17
History, physical examinations, and associated symptoms are crucial for distinguishing between various conditions. However, one notable differentiation lies in MRI imaging. Muscle injuries and inflammation typically display signal changes or edema, particularly in acute phases. Muscle herniation is characterized by protrusion from a defect. Accessory muscles and hypertrophy, on the other hand, exhibit similar MRI signals to normal muscles.8 Additionally, focal neurogenic hypertrophy can occur following radiculopathy or old poliomyelitis. Electromyography study can help diagnose the latter conditions.5
There are several treatment options available for isolated muscle hypertrophy. These options include symptomatic treatment with analgesics for patients experiencing pain, muscle reduction surgery, or BTX injection. Previous studies utilizing BTX have shown promising results in pain management and the reduction of muscle hypertrophy.18-20 BTX injection represents a simple and minimally invasive approach. The mechanism of action of BTX involves inhibiting acetylcholine release at the neuromuscular junction, resulting in muscle relaxation and a decrease in muscle hypertrophy.21, 22 BTX injection temporarily paralyzes the affected muscle, increasing the potential for a reduction in muscle hyperactivity, improved patient function, and a decrease in the frequency and severity of associated pain.23
The patient experienced significant pain relief and improved function following BTX injection, as evidenced by the reduction in pain score and improvement in the DASH questionnaire scores during follow-up visits. Physical examination also revealed a marked reduction in the size of muscle enlargement.
Overall, BTX injection appears to be a viable treatment option for idiopathic muscle hypertrophy, offering pain relief, improved function, and reduction in muscle size without reported adverse effects in this case. Further research and long-term follow-up studies are warranted to assess the long-term efficacy and safety of BTX in managing idiopathic muscle hypertrophy.
AUTHOR CONTRIBUTIONS
Sarvenaz Rahimibarghani: Conceptualization; writing – original draft; writing – review and editing. Leila Aghaghazvini: Investigation; project administration; resources. Hamid R. Fateh: Conceptualization; supervision; writing – review and editing. Niloofar Shirzad: Investigation; resources; writing – review and editing.
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to Dr. Faezeh Moghimpour-Bijani.
FUNDING INFORMATION
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST STATEMENT
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICS STATEMENT
This study was approved by the Tehran University of Medical Sciences ethical committee.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated.




