A rare case of pulmonary mucormycosis and broncho-esophageal fistula in a patient with poorly controlled diabetes
Abstract
Key Clinical Message
In patients with poorly controlled diabetes, early recognition of rare fungal infections like pulmonary mucormycosis, especially when presenting with unusual complications such as broncho-esophageal fistula, is critical. Prompt intervention with antifungal therapy and consideration for surgical debridement significantly impact outcomes. Multidisciplinary management is paramount for such complex cases.
Mucormycosis is a rare fungal infection caused by the Mucorales. This infection is mostly observed among those with poorly controlled diabetes or immunodeficiency. The most common presentation of the infection among those with poorly controlled diabetes is rhino-orbit-cerebral involvement. In this case report, we provide the history and outcome of a rare case of pulmonary mucormycosis in a patient with poorly controlled diabetes who was simultaneously diagnosed with broncho-esophageal fistula. Our patient was a 32-year-old male with a history of poorly controlled diabetes. Over the months, he had complained of productive coughs and dyspnea, which had lately been joined by dysphagia. He also claimed to have lost considerable weight (10 kg) during the previous 3 months. Barium swallow showed an abnormal flow of contrast between the bronchus and esophagus, suggesting a broncho-esophageal fistula. Computed tomography of the thorax revealed a broncho-esophageal fistula between the left main bronchus (LMB) and esophagus. He had a bronchoscopy the next day, which revealed necrosis and a broncho-esophageal fistula in the LMB. A bronchial biopsy showed typical hyphae with necrotic tissue, indicating mucormycosis. The patient's antimycotic medication (liposomal amphotericin) was started and a prompt surgery consult was ordered. The patient, however, passed away from massive hemoptysis. We described a rare case of pulmonary mucormycosis with broncho-esophageal fistula in a patient with poorly controlled diabetes. The rarity of this combination highlights the associated diagnostic and treatment hurdles. Early detection, antifungal medication, as soon as possible surgical debridement of involved tissues, and a multidisciplinary approach could improve patient outcomes.
1 INTRODUCTION
Mucormycosis, caused by a fungus of the order Mucorales, is a rare infection.1 The sites most commonly affected by the infection include the sinuses, brain, and lungs.2 The rhino-orbit-cerebral involvement was associated with uncontrolled diabetes in a study in India.3 Among the associated risk factors (including immunodeficiency), diabetes is known as the most common risk factor, and studies show that infection is on the rise among these patients.4 The infection develops rapidly and the hallmark of diagnosis is tissue necrosis.5
The presence of an abnormal connection between the esophagus and the respiratory system is referred to as esophagorespiratory fistula (ERF) which falls into two subcategories: tracheo-esophageal fistula (TEF) and broncho-esophageal fistula (BEF).6 Among the two, BEF is far less common and the current evidence is primarily derived from case reports. BEF can be acquired due to a set of reasons, including neoplasms as the most common, infections, and trauma.7 In addition to mucormycosis, previous studies have reported other pathogens responsible for causing a BEF, such as tuberculosis, candidiasis, syphilis, and histoplasmosis.8-12 On the other hand, broncho-esophageal fistula with pulmonary mucormycosis coexisting is a highly unusual presentation with few documented occurrences in the medical literature.
This case report provides the history and clinical outcome of a rare case of a patient with poorly managed diabetes who simultaneously experienced broncho-esophageal fistula and pulmonary mucormycosis. This article goes over the patient's clinical presentation, diagnostic difficulties, treatment approaches, and clinical results and hopes to raise awareness of uncommon fungal infections in people with poorly managed diabetes, stress the need for early detection, and encourage further study in this area.
2 CASE EXAMINATION
The patient we are introducing here is a 32-year-old man with a history of poorly controlled diabetes. Over the months, he had complained of productive coughs and dyspnea, which had lately been joined by dysphagia. He also claimed to have lost significant weight (10 kg) during the previous 3 months. At presentation, pulse rate was 105 beats per minute, respiration rate was 25 beats per minute, blood pressure was 140/70 mmHg, and temperature was 37.6°C. His SpO2 was 80% when the mask was ventilated, rising to 95%.
3 METHODS
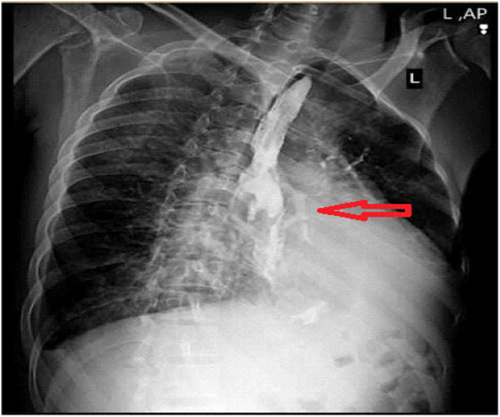
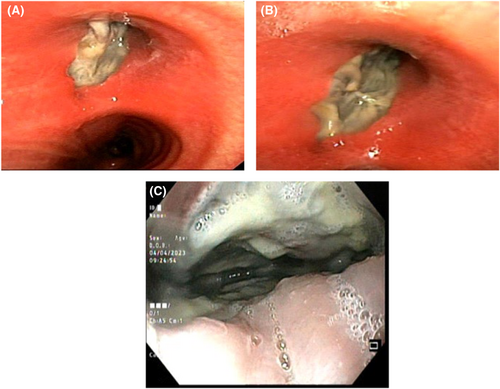
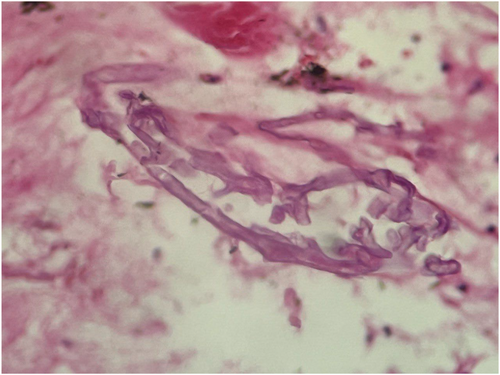
The patient's first evaluation indicated elevated blood sugar, which led to the supposition that diabetic ketoacidosis (DKA) was present. All Blood work analyses are accessible in Table 1. Therefore, the patient was treated for DKA at first, but his symptoms persisted. The barium swallow revealed an unusual flow of contrast material from the bronchus to the esophagus, indicating the presence of a broncho-esophageal fistula (Figure 1). He was subsequently transported for bronchoscopy as a result of the patient's symptoms of brown sputum and the findings of the CT scan (Figure 2). The parenchymal view findings were nonspecific, such as pleural effusion and few parenchymal opacities. Also, the PCR test was Negative. Given his grave circumstances, the patient is immediately admitted to the intensive care unit. He got meropenem and vancomycin in the ICU. He had a bronchoscopy the next day, which revealed necrosis and a broncho-esophageal fistula in the left main bronchus (LMB). The patient underwent an endoscopy, which revealed erythematous mucus, broncho-esophageal fistula, and necrosis, according to the GI consultation (Figure 3). Both endoscopy and bronchoscopy were performed on the patient. Biopsy samples were taken from the fistula site, revealing necrosis and a diagnosis of mucormycosis. Given that mucormycosis is an invasive and necrotizing infection, the broncho-esophageal fistula may have developed due to this fungal infection. Once mucormycosis was determined by the pathology report from the bronchoscopy and endoscopy, the patient's antimycotic medication (liposomal amphotericin) was started (Figure 4) and thoracic surgery consult was ordered but The patient developed life-threatening hemoptysis and left-sided double-lumen endotracheal tube was placed urgently, due to the patient's hemodynamic instability, surgery was not feasible, and the patient deceased from life-threatening hemoptysis.
| Test name | Result | Unit | Reference value |
|---|---|---|---|
| Hematology-ESR | |||
| ESR (1 h) | 67H | mm/h | <15 |
| SARS Corona virus-2 | |||
| Real-Time RT-PCR | Not detected | ||
| Urinalysis | |||
| Creatinine | 0.9 | mg/dL | 0.7–1.4 |
| Urea | 31 | mg/dL | 17–45 |
| Electrolytes | |||
| Sodium | 135 | mEq/L | 135–145 |
| Potassium | 4.8 | mEq/L | 3.5–5.3 |
| Liver function tests | |||
| AST (SGOT) | 51H | U/L | 14–20 |
| ALT (SGPT) | 15 | U/L | 7–56 |
| ALP | 243 | U/L | 44–147 |
| Total bilirubin | 0.4 | mg/dL | 0.1–1.2 |
| Direct bilirubin | 0.1 | mg/dL | <0.3 |
| Microbiology | |||
| Urine culture | No growth | ||
| Blood culture I (Bactec) | No growth | ||
| Hematology-CBC | |||
| WBC | 18.9H | ×103/uL | 4.5–11 |
| RBC | 4.61 | ×106/uL | 4.3–5.9 |
| Hb | 13.7 | g/dL | 13.5–17.5 |
| Hct | 39.7 | % | 41–53 |
| MCV | 86.1 | fL | 80–100 |
| MCH | 29.7 | pg | 27–31 |
| MCHC | 34.5 | g/dL | 34 ± 2 |
| RDW-CV | 12.4 | % | 11–15 |
| Platelet | 383 | ×103/uL | 150–450 |
| PDW | 11.2L | fL | 15.1–17.9 |
| MPV | 9.4 | fL | 7–11.5 |
| P-LCR | 20.1 | % | 15–35 |
| CBC-diff | |||
| Neut | 81.3H | % | 40–60 |
| Lymph | 13.0 | % | 20–40 |
| Mixed (Mono+Eos+Baso) | 5.7 | % | |
- Abbreviations: H, High; L, Low.

4 CONCLUSION
We described a rare instance of pulmonary mucormycosis with broncho-esophageal fistula in a patient with poorly controlled diabetes. The rarity of this combination highlights the associated diagnostic and treatment hurdles. Early detection, antifungal medication, and a prompt aggressive surgical debridement could improve patient outcomes. Further research and increased awareness of these rare fungal infections in patients with poorly controlled diabetes are warranted to improve early detection and enhance clinical management strategies.
5 DISCUSSION
As a rare yet emerging fungal infection, mucormycosis brings about high morbidity and mortality.1 Humans are infected by the fungus either through inhalation or through the inoculation of the fungus into skin abrasion and wounds.13 The infection mainly affects the rhino-orbital-cerebral sites in patients with poorly controlled diabetes, while among immunocompromised patients, the infection could turn into a pulmonary infection and further, a life-threatening and disseminated infection.14, 15 Also, various other conditions such as a history of transplantation, iron overload, trauma/burns, and malnutrition are considered the risk factors for this infection.15-17
On the other hand, previous studies have reported pulmonary infection as the presentation of mucormycosis in patients with neutropenia, leading to symptoms such as chest pain, fever, and dyspnea, due to hyphal invasion of blood vessels and subsequent hemorrhage.18-20 However, these symptoms were absent in our patient which highlights the rarity of our case, in that our patient was not immunocompromised but a poorly controlled diabetic patient whose fungal infection progressed into pulmonary involvement with a concomitant broncho-esophageal fistula, representing a unique and complex clinical scenario. The combination of these two conditions adds significant challenges to the diagnosis, management, and prognosis of the patient.
The clinical presentation of our patient included symptoms such as productive cough, dyspnea, dysphagia, and significant weight loss. These nonspecific symptoms can often be attributed to various respiratory and gastrointestinal conditions, leading to diagnostic challenges. More so, given that the infection could progress into a disseminated and invasive state in a matter of days,21 the urgency of an early diagnosis seems of paramount importance. Therefore, in the context of poorly controlled diabetes, it is crucial to consider opportunistic fungal infections, including mucormycosis, especially when there is a lack of improvement of symptoms after the initial treatment. Therefore, maintaining a high index of suspicion is essential for timely recognition and appropriate management.
The diagnosis of mucormycosis is based on the recognition of specific characteristics in histopathology, which could ideally be backed up by culture growth for determining the susceptibility of the pathogen to anti-fungal therapy.22 Furthermore, complicating the diagnosis, current non-culture-based serologic tests for invasive fungal infections are not specific to mucormycosis. They can indicate the presence of other fungal pathogens but cannot identify the specific one causing mucormycosis. No serum assays are currently available exclusively for mucormycosis.23 Furthermore, the infection can be difficult to recognize in the CT imaging at early stages, given the non-specific findings such as peribronchial ground-glass opacity. However, as the disease progresses, consolidation, nodules, or masses appear. Also, some findings, namely the reverse halo sign, help differentiate this infection from similar infections such as invasive pulmonary aspergillosis.24
Later, the disease progresses to consolidation, nodules, or masses. Various radiologic findings were suggestive of PM have been identified to help differentiate it from IPA. For example, the reverse halo sign is more closely associated with PM than with IPA. The reverse halo sign is an area of ground-glass opacity surrounded by a rim of consolidation. In addition, the presence of pleural effusions and more than 10 nodules is more suggestive of PM than it is of IPA.
The key to the mystery of the present case was solvable only through bronchoscopy which revealed the presence of a broncho-esophageal fistula in the LMB, along with necrotic tissue. The samples taken via bronchoscopy then demonstrated mucormycosis as the underlying cause of these fistulas, leading to prompt initiation of antifungal treatment with liposomal amphotericin. These findings highlight the importance of invasive diagnostic procedures to establish an accurate diagnosis and guide appropriate therapeutic interventions.
The mechanisms that make diabetes a great risk factor for the development of mucormycosis have previously been identified. The acidosis that comes with DKA makes a favorable environment for the fungus spores to reproduce. Another reason is that during hyperglycemic episodes, the iron binding capacity is decreased leading to high free iron levels, which eliminates an important part of the defense mechanisms.25 However, what could be the underlying mechanism of developing a BEF in this case? The coexistence of pulmonary mucormycosis and broncho-esophageal fistula is exceptionally rare, with limited reported cases in the medical literature. The pathogenesis of this association is not fully understood, but it is postulated that the angioinvasive nature of mucormycosis and the necrosis observed in histologic examination may contribute to the erosion of adjacent structures, leading to the formation of fistulas.26 Comparing our case to the findings by Kumar and Joshi,27 who documented mucormycosis in immunocompetent individuals, underscores the infection's broad impact, challenging the notion that it primarily affects those with significant immunocompromises. Our research, coupled with Kumar and Joshi's findings, highlights the necessity of considering mucormycosis in varied clinical scenarios and advocating for vigilant diagnostic practices to facilitate timely treatment interventions.
Treatment of mucormycosis involves a combination of surgical debridement of involved tissues and antifungal therapies. Prompt antifungal treatment and aggressive surgical debridement for the BEF should be devised as soon as possible.28 If left untreated or received treatment with a delay, these patients have minimal chance of surviving.
Although this case showed the devastating consequences of pulmonary mucormycosis with broncho-esophageal fistula, it highlighted the fact that no matter how rare this condition is, it needs attention.
AUTHOR CONTRIBUTIONS
Farid Poursadegh: Project administration. Safieh Shazdeh Ahmadi: Supervision; writing – original draft. Zahra Oskouyan: Supervision; writing – original draft. Mohammad Mahdi Alvandi Fard: Writing – review and editing. Fariba Rezaeetalab: Supervision. Mahnaz Mozdorian: Supervision. Reza Basiri: Supervision.
ACKNOWLEDGMENTS
We would like to acknowledge the invaluable contribution of Dr. Reza Basiri during the revision process of this article which significantly enriched the content and quality of this study. Due to the limitations of the author panel, we kindly request the editorial team to include Dr. Reza Basiri ([email protected]) as a co-author of this article.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
The author declares that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.




