Favipiravir-induced bradycardia: A case report
Alireza Kashefizadeh, Laya Ohadi, and Farbod Amiri shared first co-authorship.
Abstract
Key Clinical Message
The purpose of this case report is to reveal one of the cardiovascular side effects of favipiravir, sinus bradycardia.
Favipiravir has emerged as a potential treatment for COVID-19, with its antiviral properties showing promise in inhibiting viral replication. However, concerns regarding its safety profile, particularly its cardiac adverse effects, remain a subject of debate. We present the case of a 58-year-old man with a history of diabetes mellitus and chronic obstructive pulmonary disease who developed bradycardia following treatment with favipiravir for COVID-19 pneumonia. Despite being asymptomatic, the patient exhibited sinus bradycardia, which resolved upon discontinuation of favipiravir. Favipiravir has been associated with QT prolongation and sinus bradycardia, though the exact mechanisms remain unclear. Our case adds to the growing body of evidence highlighting the potential cardiac complications of favipiravir therapy in COVID-19 patients. Further research is warranted to clarify the underlying mechanisms and optimize patient management strategies. Clinicians should be cautious for cardiac adverse events when prescribing favipiravir for COVID-19 treatment, especially in patients with preexisting cardiac conditions. Continued research is essential to ensure the safe and effective use of favipiravir in the management of COVID-19.
1 INTRODUCTION
Among the drugs discovered to treat pneumonia-19, antiviral drugs such as remdesivir and favipiravir play an important role in counteracting acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by preventing virus replication and disease progression.1 Prior to the availability of the current COVID-19 vaccine, the global focus on pandemic control centered on utilizing approved antivirals. Favipiravir presented itself as a potential treatment for COVID-19, leveraging its proven efficacy against diverse viral infections.2 Favipiravir, originally introduced in Japan as an anti-influenza medication in 2014, exhibits potential as an antiviral drug, with applications in treating Ebola and various viral illnesses. Functioning as a nucleoside analog, it interacts with the RdRp complex of SARS-CoV-2, possibly elevating mutation frequency and inducing lethal mutagenesis.3
The prescribed amount of favipiravir administered varies depending on the indication. For COVID-19 treatment, higher doses are typically advised. As per the COVID-19 treatment guidelines provided by the Ministry of Health, a dose of 1600 mg of favipiravir is recommended twice daily on the initial day of treatment. Subsequently, patients with pneumonia and probable/definitive disease are advised to take 600 mg twice daily for 5–10 days.4 Like other drugs, favipiravir can cause side effects, but its side effects are usually tolerable and most of them are related to the gastrointestinal tract such as nausea, vomiting, diarrhea, and abdominal discomfort.5 While favipiravir is typically considered a safe drug, it can occasionally lead to adverse cardiac effects such as QT prolongation.6, 7 In terms of overall and serious adverse events (AEs), favipiravir exhibits a favorable safety profile. Yet, certain safety aspects like hyperuricemia, teratogenicity, and QTc prolongation require further investigation. While short-term use of favipiravir appears safe and tolerable, additional evidence is crucial for assessing its potential long-term effects.8
Let us now introduce another effect of favipiravir on the cardiovascular system, which is bradycardia. The exact mechanism of this outcome is not yet known, and further studies are needed to elucidate this mechanism.
2 CASE PRESENTATION
A 58-year-old man presented to emergency department with a three-day history of fatigue and progressive shortness of breath (SOB). He had no complaints of other symptoms including fever, headache, dry cough, and chest pain. His past medical history (PMH) was positive for diabetes mellitus (DM) type 2 and chronic obstructive pulmonary disease (COPD) since 10 and 5 years ago, respectively. Drug history consisted of metformin 500 mg daily and he did not have any medication for COPD. Patient's stable hbA1C levels, along with consistent adherence to metformin therapy and lifestyle adjustments, provide reassuring evidence regarding the management of his type 2 DM. His initial vital signs were stable (blood pressure: 130/90 mm of mercury, pulse rate: 84/min, respiratory rate: 18/min, and temperature: 36.8°C) except oxygen saturation (O2sat) that was 78%. He was admitted as a possible case of COVID-19 pneumonia due to progressive SOB and low O2sat.
3 METHODS
Diagnostic investigations were performed and computed tomography (CT) scan of the lungs displayed mild to moderate bilateral patchy infiltration that is seen in COVID-19 pneumonia. Moreover, the polymerase chain reaction (PCR) test was positive for SARS-CoV-2.
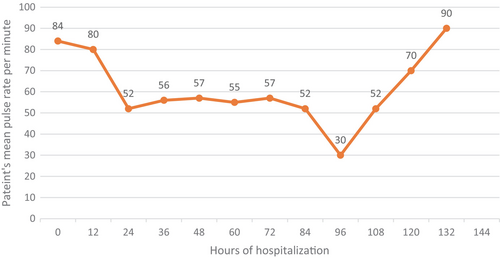
Instantly, treatment according to the protocol was started for this patient including oral favipiravir, oral vitamin C and intravenous methylprednisolone. Prior to the initiation of favipiravir treatment, an electrocardiogram (ECG) was conducted, yielding no abnormalities. Throughout the patient's hospitalization period, constant monitoring was implemented, yet no arrhythmias were observed. Interestingly, this patient developed bradycardia (52/min) 24 h after admission and his pulse rate decreased as revealed in Figure 1, reaching 30/min (4th day of admission). We evaluated the patient for any symptoms related to bradycardia, but he was symptom free. Sinus bradycardia was detected in his electrocardiogram (ECG) as well. Patients mean pulse rates are demonstrated in Figure 1.
Laboratory tests were within the normal range as demonstrated in Table 1. Electrolytes, thyroid hormone levels, and cardiac enzymes were within normal limits. He was well without any complaints, but full cardiac monitoring was done for him for accurate assessment. Despite the patient's bradycardic episode and the potential association with the medication, no significant alterations in QTc, QRS duration, QRS axis change, and T wave change parameters were noted. He denied taking any medication such as beta blockers. In addition, no history of heart disease was noticed.
| Markers | Value w/units | Normal range |
|---|---|---|
| Urea | 4.5 mmol/L | 2.8–8.1 |
| Creatinine | 1.08 mg/dL | 0.7–1.3 |
| Sodium | 139 mmol/L | 136–145 |
| Potassium | 4.3 mmol/L | 3.5–5.1 |
| White blood cell count | 8.3 × 103/uL | 4.0–10.0 |
| Hemoglobin | 15.3 gram/dL | 13.2–16.6 |
| Platelet count | 400 × 103/uL | 150–400 |
| Calcium | 9.4 mg/dL | 8.6–10.3 |
| Magnesium | 0.80 mmol/L | 0.66–1.07 |
| Troponin | 0.01 ng/mL | 0–0.04 |
| LDH | 175 U/L | 140–280 |
| Aspartate aminotransferase | 11 U/L | 10–40 |
| Alanine aminotransferase | 17 U/L | 7–56 |
| Alkaline phosphatase | 120 U/L | 30–120 |
| Creatine phosphokinase | 263 U/L | 39–308 |
| HbA1c | 5.3% | <5.7% |
| TSH | 1.1 mIU/L | 0.5–5.0 |
4 CONCLUSION AND RESULTS
We supposed that bradycardia is a complication of inpatient medication. Therefore, favipiravir was suspended at first and we found that his pulse rate increased, reaching 70/min on day five and stabilized between 70 and 90/min. Additionally, since the patient's bradycardia resolved following the discontinuation of favipiravir, and there were no signs or history of heart disease, there was no indication for performing an echocardiogram. Considering other potential underlying causes of the observed bradycardia, we initially discussed the possibility of amyloidosis given its association with cardiac conduction abnormalities. However, we acknowledge that amyloidosis was not evaluated during the patient's hospital stay for COVID-19 as there were no other related signs and symptoms. Consequently, sinus bradycardia is a probable diagnosis induced by favipiravir in our patient.
5 DISCUSSION
Favipiravir is known as an antiviral drug with inhibitory influence that affects ribonucleic acid (RNA) polymerase.9 Despite the extensive use of favipiravir during the COVID-19 pandemic, there is limited information regarding the favipiravir's cardiac complications.10 One of the most important cardiac side effects is QT prolongation that initially was reported in an Ebola virus-infected patient who was treated with favipiravir.11 In a study on cardiomyoblast cell lines, it was concluded that favipiravir can impact cellular functions, including increased oxidative stress and DNA damage, suggesting potential harm to cardiac health.12 Furthermore, chest pain was detected by prescription of this drug.13
Another effect of this drug is sinus bradycardia that means reduced heart rate, reaching lower than 60 bpm with a normal p wave. The symptoms varied from asymptomatic individuals to chest pain and syncope.14 During our patient's hospitalization, he was not given medications that cause bradycardia such as betablockers, calcium channel blockers, digoxin and ivabradine. Besides, no underlying diseases were detected in this patient including ischemic heart disease, hyperkalemia, hypothyroidism, and amyloidosis.15 After stopping favipiravir, his pulse rate increased and reached the normal range. These features support the transient bradycardia induced by favipiravir theory that previously displayed. Moreover, Habib et al. reported a 57-year-old woman who developed sinus bradycardia, heart rate reaching to 30 bpm, induced by favipiravir. In addition, heart rate reached to normal range when favipiravir was stopped and dexamethasone (a possible cause of bradycardia) continued.14 Szigeti demonstrated transient sinus bradycardia induced by favipiravir in another patient as well.16 Due to lack of information about the mechanism of action, novel control trials are needed to prove this effect and reveal the mechanism.
Our case presentation highlights a significant observation of bradycardia in a patient treated with favipiravir for COVID-19 pneumonia. Despite being generally well tolerated, favipiravir can occasionally induce cardiac complications such as QT prolongation and sinus bradycardia. In our patient, sinus bradycardia resolved upon discontinuation of favipiravir, suggesting a probable association between the drug and the observed cardiac effect.
While further research is needed to clarify the exact mechanisms underlying favipiravir-induced bradycardia, our findings underscore the importance of monitoring for cardiac adverse events in patients receiving favipiravir therapy. Clinicians should remain cautious, especially when prescribing higher doses of favipiravir, and consider alternative treatment options in patients with preexisting cardiac conditions.
In summary, our case contributes to the growing body of evidence regarding the cardiovascular safety profile of favipiravir and emphasizes the need for continued surveillance and research to ensure the safe and effective use of this antiviral agent in the management of COVID-19.
AUTHOR CONTRIBUTIONS
Alireza Kashefizadeh: Supervision; writing – original draft; writing – review and editing. Laya Ohadi: Supervision; writing – original draft; writing – review and editing. Farbod Amiri: Supervision; writing – original draft; writing – review and editing. Maryam Abdolmaleki: Writing – original draft; writing – review and editing. Vahid Eslami: Writing – original draft; writing – review and editing. Mehrdad Jafari Fesharaki: Writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
None to declare.
FUNDING INFORMATION
No additional funding for the execution of the present study was received.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing or conflict of interests.
ETHICS STATEMENT
The patient consent has been taken and consent form is with the editor and available on request.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Research
DATA AVAILABILITY STATEMENT
The data and materials used in the current study are available from the corresponding author on reasonable request.




