18F-FDG PET/CT in early phase of sporadic Creutzfeldt-Jacob disease: A case report
Abstract
Key Clinical Message
Creutzfeldt–Jakob disease is a neurodegenerative disorder caused by brain accumulation of a misfolded form of the cellular prion protein, whose diagnosis is challenging, particularly in early stages, due to the variability and nonspecificity of the clinical and radiological features. 18F-fluorodeoxyglucose positron-emitted tomography has the potential to be considered a crucial investigation in these patients, revealing metabolic abnormalities earlier than the conventional neuroimaging analysis.
A 59-year-old man, the military officer, was referred to our Units for the onset of neurological symptoms rapidly evolving within a month, characterized by akinetic mutism, constructional apraxia, and disorders of spatial orientation. Brain 18F-fluorodeoxyglucose (18F-FDG) positron-emitted tomography (PET)/CT depicted an asymmetric hypometabolism in the left fronto-temporo-parietal cortex, as well as in the left thalamus and the right cerebellar hemisphere, while the glucose metabolism appears to be preserved in the somatosensory cortex and the basal ganglia. Laboratory routine analyses, cerebrospinal fluid routine, infective tests, electroencephalography (EEG), and brain magnetic resonance (MR) were all unremarkable. A positive RT-QuIC result on cerebro-spinal fluid (CSF) was subsequently shown, without any pathogenic gene mutations and, therefore, the result was consistent with a diagnosis of sporadic Creutzfeld–Jacob disease. The clinical evolution was quickly unfavorable, and the patient died about 4 months after hospital admission. FDG PET/computed tomography (CT) has the potential to be considered a crucial investigation in these patients, documenting metabolic changes long time before other diagnostic investigations such as CSF, EEG, brain CT, and brain MR, thus suggesting a greater sensitivity of glucose metabolic evaluation in the early stage of the disease in question.
1 INTRODUCTION
Creutzfeldt–Jakob disease (CJD), a neurodegenerative disorder caused by brain accumulation of a misfolded form of the cellular prion protein, represents the most common form of transmissible spongiform encephalopathy. This group of diseases, first identified in animals in the 17th century (sheep's scrapie), is rare in humans; its frequency is around 1 in a million per year, with equal distribution worldwide.1 CJD includes a sporadic Creutzfeld–Jacob disease form (sCJD) of unknown etiology, which is epidemiologically prevalent. It is a presenile dementia lasting a few weeks before the appearance of ataxia, myoclonia, and pyramidal and extrapyramidal signs; its symptoms can be similar to those of Alzheimer's disease but with a faster worsening, which rapidly leads to death. Most sporadic cases of sCJD occur in adults between the ages of 45 and 75 years; the symptoms develop meanly between the ages of 60 and 65 years. The diagnosis is challenging, particularly in early stages, due to the variability and nonspecificity of the clinical and radiological features. The clinical onset is like other forms of dementia, with the fast worsening as the only element of suspicion; electroencephalography (EEG) recordings remain unspecific until the onset of the typical periodic triphasic waves, which, however, corresponds to a phase of the disease with a clear clinical diagnosis. Furthermore, brain magnetic resonance (MR) can remain unspecific until the death of the patient. Despite the numerous diagnostic tools, mainly based on neuroimaging techniques, there is limited information regarding the metabolic alterations in this type of pathology.2
Brain cortical 18F-fluorodeoxyglucose (FDG) uptake can help in the diagnosis in addition to brain MR, EEG, and cerebro-spinal fluid (CSF) analysis, however, such biomarkers showed suboptimal diagnostic accuracy. FDG positron-emitted tomography (PET) with computed tomography (CT), indeed, can anticipate other pathological findings and can lead to the right diagnosis.
We report the case of a patient with sCJD, in the early stage of the disease, where PET-CT revealed wide brain alterations unlike other diagnostic investigations, which were all unremarkable.
2 CASE HISTORY/EXAMINATION
A 59-year-old Caucasian man was referred to the Neurology Unit of our Public Hospital, after a neurological outpatient visit. He lived with his second wife and worked as a military officer; there was no family history of neurological diseases, no drug or alcohol use, and no recent travel, diseases, or hospitalizations. One month before the beginning of his symptomatology, he received the second dose of anti-Covid-19 viral-vector vaccine.
The wife and his sons worried about the onset of symptoms characterized by akinetic mutism, constructional apraxia, and disorders of spatial orientation, rapidly evolving within a month and causing, moreover, his work absence; the symptoms were initially considered mood disorders, even if there were no clear causes for it, but afterword, he developed a progressive cognitive dysfunction.
During hospitalization, which lasted almost 3 weeks, the patient's condition rapidly worsened: he presented delirium and hallucinations, the cerebellar ataxia worsened so that he became unable to walk, erratic myoclonus appeared as well as rigidity.
3 METHODS (DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT)
When the patient was admitted to the Neurological Unit, physical examination was normal; neurological examination showed mild gait ataxia with multidirectional oscillations, above all the cognitive symptoms previously described.
The recommended initial screening tests, for the evaluation of rapidly progressive dementia, were performed (see Table 1 for the main results). These included blood count, a basic metabolic panel including magnesium level, liver function tests, erythrocyte sedimentation rate, antinuclear antibody, C-reactive protein, VES, thyroid function tests, vitamin B-12, HIV, Lyme disease titer, autoimmune antibodies, and urine analysis.
| Test | Serum | Cerebrospinal fluid |
|---|---|---|
|
Oncomarkers Ferritin, alpha-feto protein, Ca 125, Ca 19.9, Cyfra 21.1, C.E.A., neuron-specific enolase (NSE), prostatic-specific antigen |
All normal except for NSE: 20.5 (<17) | Not performed |
|
Neural autoantibodies (immunofluorescence) NMDA-R, AMPA-R 1–2, CASP-R2, LGL1, DPPX, GABA-R, Amphiphysin, CV2, PNMA2 (Ma-2/Ta), Ri, Yo, Hu, Recoverin, SOX1, Titin, Zic4, GAD65, DNER |
Absent | Absent |
| Antiacquaporin, and anti-MOG antibodies | Absent | Absent |
|
Quantitative DNA HSV1, HSV2, CMV, EBV |
Absent | Absent |
| VDRL, HIV 1-2antigens and antibodies | Negative | Not performed |
All the exams' results were normal, allowing us to exclude autoimmune encephalitis, paraneoplastic and toxic-metabolic encephalopathies.
Brain MR was performed with a Siemens-Magnetom Aera, of 1.5 T, with SE T1, SE T2, and DWI sequences. The thickness of sections was about 4 mm.
The patient underwent an FDG-PET brain imaging at our Department of Nuclear Medicine following a fasting of 6 hrs. After intravenous administration of 185 MBq activity of FDG, the patient rested in a quiet dark room with the eyes closed for 30 min. FDG-PET was performed with a “GE-Discovery IQ” PET/CT scanner, with CT low-dose attenuation correction. The images were reconstructed using a 400 × 400 matrix with voxel size 1.02 × 1.02 × 3 mm and 4 mm and Gaussian filter using OSEM-QClear algorithm. The images of Brain 18F-FDG PET/CT were analyzed, both qualitatively and semi-quantitatively, using “Cortex ID software” GE Healthcare.
No specific treatments were administrated to the patient.
4 CONCLUSION AND RESULTS (OUTCOME AND FOLLOW-UP)
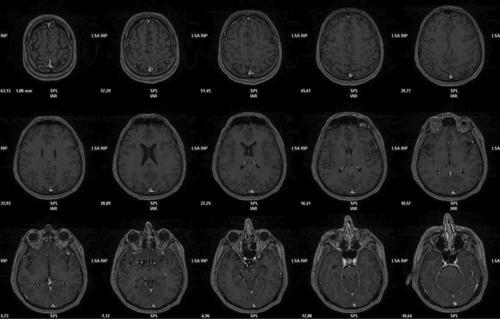
Results of initial screening tests demonstrated no relevant abnormalities. Brain MR was unremarkable (Figure 1): diffusion-weighted imaging did not show cortical diffusion-restriction, neither in basal ganglia, thalamus, and cortex. The “hockey stick” or “pulvinar” sign was also not revealed.
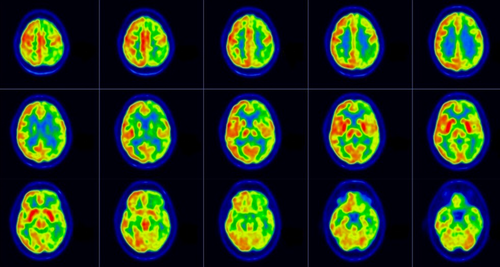
Brain 18F-FDG PET/CT transaxial images (Figure 2) showed an asymmetric hypometabolism in the left fronto-temporolateral-parietal-occipital cortex, as well as in the left thalamus and the right cerebellar hemisphere, while the glucose metabolism appears to be preserved in somatosensory cortex and basal ganglia.
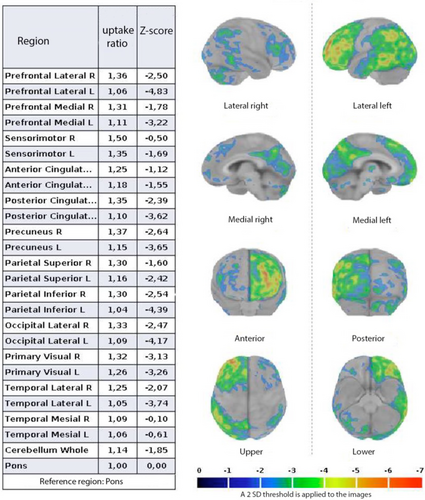
Quantitative analysis by Cortex ID (Figure 3) confirmed the above-mentioned qualitative.
The cerebrospinal fluid routine and the infective tests (see Table 1) were unremarkable.
A positive RT-QuIC result on CSF was subsequently found, without any pathogenic gene mutations, with codon 129 (MV) MET/MET polymorphism and, therefore, compatible with sCJD. The above analysis was performed by Neuroscience Department-Istituto Superiore di Sanità Rome, Italy.
Clinical evolution was quickly unfavorable. The patient died about 4 months after hospital admission. The fact that the patient died early was related to a rapid disease progression. It is known that sCJS usually leads to death within 1 year. In literature, it is described that basal ganglia involvement is related to a rapid disease progression,3 but we did not observe this in our case.
5 DISCUSSION
Cognitive impairment is a common presentation of many neurological diseases, consequently with a wide differential diagnosis.
Prion diseases are a very rare cause of dementia, and their recognition is very hard to detect, particularly in early stages, when the laboratory and the radiological exams do not present significant alterations, and the suspicion can be only based on the clinical presentation.
MR diffusion-weighted imaging (DWI) has been reported for the higher sensitivity of for the initial diagnosis of sCJS.4
The reason is that the spongiform change (i.e., vacuolization) occurs before neuronal loss and seems to restrict the water diffusion, leading to the hyperintensity of the lesions on DWI.5
Hyperintensity DWI lesions are known to appear in the cerebral cortex and/or basal ganglia and then to progress to diffuse brain atrophy over weeks or months.6
Magnetic resonance spectroscopy (MRS) provides potentially useful information about brain metabolites such as N-acetylaspartate (NAA). NAA can be considered a surrogate marker of the neuron, and its level reflects the neuronal density. Although MRS studies are limited, reduced NAA levels have been reported in affected cortical regions.7, 8 However, no significant NAA decline has been found at early stage of thedisease.9
This is consistent with the literature describing that the spongiform change predominates at the early stage of sCJS, whereas the neuronal loss occurs afterward.10
Another diagnostic nuclear imaging is represented by SPECT with a variety of radionuclides such as 99mTc- hexamethylpropyleneamine oxime and 99mTc- ethyl cysteinate dimer (ECD) that are used in the evaluation of cerebral perfusion. They have been used in the last two decades for differential diagnosis of several dementias, and the images obtained are often similar.11
Several case reports showed that SPECT abnormalities were revealed in early phase unlike other techniques documented normal findings.12, 13
Many investigators have used SPECT for the evaluation of sCJS, which often reveals a heterogeneous reduction of the cerebral regional blood flow throughout the brain.14, 15
In addition, Kirk et al. have reported that abnormalities in SPECT correlate with areas of spongiform change and neuronal loss.16
The choice of type of nuclear diagnostic investigation also depends on the availability of various hospital centers; obviously, where PET is available, this one is preferable to SPECT for its higher image resolution and diagnostic accuracy.
Classical topographic distribution patterns of cerebral hypometabolism usually may help in formulating the diagnosis of the major neurodegenerative pathologies in a predementia phase, such as mild cognitive impairment, but are not univocal in sCJD.
Together with the complexity of the clinical features, the radiodiagnostic findings can be variable too, and sometimes they lack typical findings.17-22
Many studies in the literature reported different imaging features about cortical and subcortical FDG uptake that sometimes are discordant with respect to brain MR findings,22-25 which, however, can guide the diagnosis of sCJD.
The scientific literature reports cases of patients affected by sCJD with patterns of glucose hypometabolism in frontal, parietal, and occipital cortices of both hemispheres, in the middle temporal-gyrus, and superior temporal-gyrus with a right-sided prevalence.19 An asymmetrical cortical distribution pattern of 18F-FDG is often reported,17 which can be mistaken for a cortical-basal-degeneration (CBD)22; in CBD, there is often an involvement of the basal ganglia, just unlike our case.26 However, in sCJS with clinically a cortico-basal syndrome presentation, the basal ganglia may be involved.27-31 A primary cortices involvement is also described in CJS31 and rules out the diagnosis of neurodegenerative syndromes such as Alzheimer's disease and Lewy Body degeneration, conditions in which such areas are usually spared.32 Several studies have also shown low involvement of the medial-temporal areas, thus suggesting a possible resistance of hippocampus toward prion deposits.33, 34
Although the mechanisms underlying the spread and infectivity of prions are currently well understood, the determinants of prion neurotoxicity and pathogenesis are also not fully understood.35
FDG PET/CT can be potentially considered a crucial investigation in these patients because it reveals metabolic changes long time before other diagnostic methods, such as CSF, EEG, CT, and MR, thus suggesting a greater sensitivity of glucose metabolic evaluation in the early stage of this disease.
We can hypothesize that in the early stage of sCJD the initial neuronal disfunction and the microcirculatory alterations, that occur before the gray matter volume loss (brainMR T1 sequences), can be detectable only by 18F-FDG PET/CT scan.
Therefore, we believe that patients with suspected sCJD should be regularly evaluated from a metabolic point of view, especially in early stages, when classical clinical criteria and diagnostic investigations lead to non-univocal results.
Obviously, a larger number of patients is needed to better analyze the relationship between early clinical presentation and FDG-PET metabolism.
6 CONCLUSION
Our study has some limitations. First, we did not have the opportunity to evaluate the evolution of the metabolic changes in this patient as he died in a short time. It would have been interesting to follow up on the progression of the disease.
Another limitation, as previously reported, is the lack of histopathological autoptic validation, although positive RT-QuIC results on CSF confirmed sCJD diagnosis.
In this context, it would be interesting the availability, in the future, of a software application for PET images postprocessing in order to guide the diagnosis in a more objective way, comparing the images obtained with a large database of patients affected by sCJD.
Based on this consideration, we think that the findings of our case report provide useful information regarding the advantage of 18F-FDG PET in revealing metabolic abnormalities earlier to the conventional neuroimaging analysis, such as brain MR, and, in addition, it may help to improve the understanding of the biomolecular processes underlying this type of neurological disorders.
AUTHOR CONTRIBUTIONS
Giovanni Boero: Writing – original draft. Filippo Lauriero: Validation. Donato Fusillo: Investigation. Rosa Calvello: Visualization. Antonia Cianciulli: Visualization. Maria Antonietta Panaro: Supervision. Piergianni Moda: Formal analysis.
FUNDING INFORMATION
This research received no external funding.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to be disclosed.
ETHICS STATEMENT
Not applicable.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
All data are reported in the manuscript.




