Prenatal diagnosis of cystinuria with a heterozygous pathogenic variant in SLC7A9 gene associated with isolated hyperechogenic fetal kidneys: A case report
Key Clinical Message
Cystinuria is suspected antenatally by a hyperechogenic fetal colonic content. We report the first prenatal case of autosomal dominant SLC7A9-related cystinuria associated with isolated hyperechogenic kidneys as the only prenatal sonographic sign.
1 INTRODUCTION
Urinary tract anomalies represent approximately 20% of the fetal malformations.1 Hyperechogenic kidneys are defined as a renal parenchyma more echogenic than the adjacent liver or spleen. It is encountered in 1.6 out of 1000 prenatal sonographic examinations.2
Hyperechogenic fetal kidneys have several etiologies such as metabolic diseases (like idiopathic infantile hypercalcemia),3, 4 congenital viral infection,4, 5 renal tubular dysgenesis, and congenital nephrotic syndromes.4, 6, 7 Fetal hyperechogenic kidneys can also be associated with congenital anomalies of the kidney and the urinary tract (CAKUT),8 autosomal dominant and recessive polycystic kidney disease (ADPKD and ARPKD), and genetic anomalies such as HNF1β mutation.9-11
The etiology and prognosis of hyperechogenic kidneys remain challenging.12-15 However, the presence of oligohydramnios is an associated poor prognostic factor.1, 12-14
Cystinuria is a hereditary tubulopathy characterized by insufficient reabsorption of dibasic amino acids (COLA: cystine, ornithine, lysine, arginine); it is associated with increased urinary excretion of COLA and possible lithiasis formation in early childhood and adolescence, which leads to progressive deterioration of renal function in 80% of patients.16-18
We report an atypical prenatal case of autosomal dominant SLC7A9-related cystinuria associated with isolated hyperechogenic kidneys. To our knowledge, only prenatal cases with hyperechogenic colon (HEC) have been reported in this context.19-21
2 CASE HISTORY
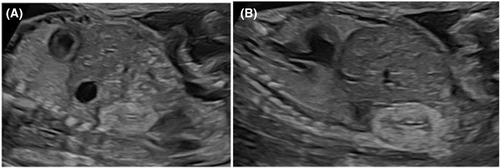
A 36-year-old Caucasian woman was referred at approximately 22 weeks of gestation for isolated hyperechogenic kidneys. The fetus was eutrophic for gestational age (Figure 1).
An early ultrasound screening at 12 weeks and 5 days was reported normal. The noninvasive prenatal testing, known as NIPT, was offered as part of the first trimester screening for Down syndrome, trisomy 13 and 18, and was normal for a female fetus.
The patient did not present any health problem, her body mass index was within the normal range, and she did not develop gestational diabetes. She did not use tobacco, alcohol, or drugs. The patient was not immunized against toxoplasmosis or cytomegalovirus (CMV).
The patient had two previous uneventful singleton pregnancies, with the same partner. The couple is not related. The first two children are two boys, one born by cesarean section for breech presentation, the second born by normal delivery. The first boy is in good health. The second still benefits from neurological follow-up for a suspicion of hereditary spastic paraplegia inherited from the father (walking on toes).
3 METHODS
For this pregnancy, the discovery of bilateral normal-sized hyperechogenic kidneys led to diagnostic hypotheses of a metabolic disease, a HNF1β mutation, or an infectious nephropathy (CMV).
The fetal medicine department team proposed an amniocentesis to the couple, for prenatal genetic and microbiological analyses, which was carried out at 22 weeks and 6 days, after parental consent. The CMV screening was unfortunately not performed in the amniotic fluid. However, the maternal serologies were negative and at birth, the CMV serology of the umbilical cord blood sampling was also negative. The quantitative fluorescence polymerase chain reaction (PCR) for chromosomes 13, 18, 21, X, and Y and the array comparative genomic hybridization excluded chromosomal abnormalities and confirmed a female fetus.
Trio clinical exome sequencing (fetal and parental DNA samples) identified the presence, at the heterozygous state, of the c.313G>A p. (Gly105Arg) variant in the SLC7A9 gene, inherited from the mother. This variant has been reported several times in the literature in families with autosomal dominant cystinuria with variable expressivity.22 It is present in population databases at a frequency of 0.05% at the heterozygous state.23 Functional testing demonstrated that this variant leads to an almost complete loss of transport activity.24 According to the American College of Medical Genetics and Genomics guidelines, this variant has been classified as pathogenic.25
Besides the genetic screening, the couple underwent a renal ultrasound, which turned out to be normal. A maternal urine analysis demonstrated an excessive excretion of COLA corresponding to a diagnosis of cystinuria. The mother never had urolithiasis, while the maternal grandfather had several episodes of urolithiasis. He declined genetic testing.
At 32 weeks and 6 days, cortico-medullary differentiation (CMD) appeared associated with hyperechogenicity of the apex of the pyramids, which were hypothesized to correspond to intratubular deposits.
A monthly fetal ultrasound monitoring until 36 weeks of gestation was proposed and accepted by the couple. At 35 weeks and 6 days, the kidneys were still hyperechogenic, undifferentiated, and growing normally with a bipolar diameter of 30 mm. During the follow-up, the bladder and the amount of amniotic fluid remained within normal values. No other morphological abnormality was identified, including the fetal colon (Figure 2).

4 CONCLUSIONS AND RESULTS
The pregnancy continued uneventfully. The patient was admitted for spontaneous labor at 39 weeks and 6 days of gestation and had a nonassisted vaginal delivery of a live female infant of 3190 g with a body length of 48 cm. Apgar was 9/10/10, arterial pH was 7.23, base excess was −3.9 mEq/L, and lactate was 3.5 mmol/L.
The neonatal clinical examination was normal. Renal ultrasound performed at 3 days of age demonstrated the presence of hyperechogenic areas at the apex of the pyramids.
At 1 month of age, a renal ultrasound showed a significantly increased medullar hyperechogenicity (Figure 3). Liquid chromatography tandem mass spectrometry confirmed an increased urinary excretion of COLA consistent with the diagnosis of cystinuria. However, the newborn presented a good clinical course with no evidence of urinary complaints.
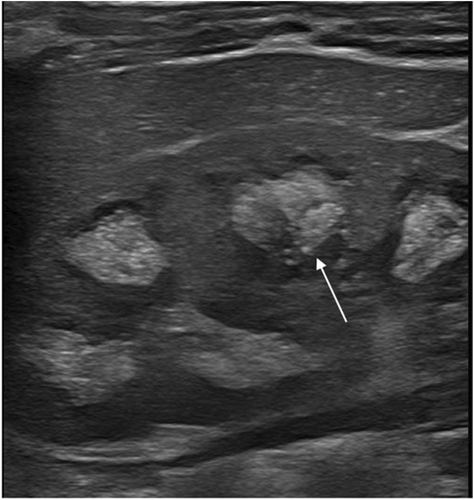
A follow-up with a nephropediatrician and a pediatric radiologist was organized. The child was monitored by renal ultrasound every 3 months during the first year of life and every 6 months during the second year of life. The last ultrasound scan performed at 2 years of age showed kidneys with good bilateral renal trophicity but persistent reversed corticomedullar differentiation. Until now, the child has been asymptomatic with a normal renal function.
5 DISCUSSION
On prenatal ultrasound, the renal pattern should present a CMD from the 18th week of pregnancy, with an echogenic cortex and hypoechoic medulla. It is important to note that this cortical echogenicity diminishes progressively throughout pregnancy.4, 17, 26, 27 The diagnosis of hyperechogenic kidneys can be made after 18 weeks of pregnancy, when the cortical echogenicity of the kidneys is higher than that of the spleen and liver.28 This diagnosis must be followed by a careful sonographic examination of the whole fetus to exclude signs of metabolic diseases or associated anomalies like CAKUT. The prenatal workup includes detection of congenital infections and genetic testing. Additional magnetic resonance imaging is not required for renal parenchymal anomalies.29
In our case, aneuploidies, such as trisomies 21, 13, and 18, which may present with hyperechogenic kidneys associated with cerebral, cardiac, and genital malformations,18, 28 had already been excluded by NIPT.
A PCR on the amniotic fluid could have been carried out to exclude a CMV infection possibly responsible of hyperechogenic kidneys. Although this is not the most common sign.5 The most frequently encountered prenatal signs are intrauterine growth restriction, cerebral ventriculomegaly, microcephaly, intracranial calcifications, fetal hydrops, oligohydramnios or polyhydramnios, hyperechogenic bowel, and hepatic calcifications.8 However, none of these signs have been observed in our case, and the umbilical cord blood sampling at birth confirmed the absence of congenital CMV infection.
Autosomal dominant and recessive polycystic kidney disease has also been ruled out from our differential diagnosis as they are hepatorenal diseases characterized by enlarged and hyperechogenic kidneys. ARPKD is associated with oligohydramnios and kidneys without CMD, and ADPKD is associated with kidneys with an increased CMD.12, 30 HNF1β mutation, which is the most common cause of hyperechogenic kidneys, was excluded by genetic testing.9, 10
Infantile hypercalcemia is a metabolic disease, also associated with hyperechogenic kidneys. It is caused by autosomal recessive variants in the CYP24A1 or SLC34A1 genes, and leads to the development of nephrocalcinosis.3, 4, 31 Genetic testing enabled us to rule out these etiologies.
As stated above, genetic testing is a key element in diagnostic investigations of hyperechogenic kidneys and in prognostic assessment.32 In our case, it showed the presence of the variant c.313G>A p. (Gly105Arg) in the SLC7A9 gene, in heterozygous state, that the fetus and the mother both carry. Given the family history and the genetic results, the diagnosis of autosomal dominant SLC7A9-related cystinuria has been retained. A urine analysis of the newborn and the mother confirmed an excessive excretion of cystine and dibasic amnio acids, although both subjects are asymptomatic to this date. Individuals with cystinuria can present with cystine urolithiasis, which manifests from childhood or adolescence, with a potential deterioration in renal function.2 This tubulopathy is characterized by a defect in tubular reabsorption, leading to abundant urinary excretion of COLA-type dibasic amino acids, such as cystine, ornithine, arginine, and especially lysine. The prevalence of this pathology is 1/7000 and the average age of first kidney stone is 13.33 The reason why urolithiasis begins postnatally in childhood is that the pH of fetal urine is +/− 7, preventing cystine stones formation. During childhood, the pH decreases, giving rise to the clinical manifestations of urolithiasis.1, 2 Diagnosis is based on microscopic examination of a stone, the observation of cystine crystals in morning urine or the high concentration of COLA in 24-hour urine and/or the ratio of COLA to creatinine.34
Mutations in the SLC3A1 and SLC7A9 genes are implicated in autosomal dominant and recessive forms of cystinuria. Autosomal dominant transmission is associated with incomplete penetrance. The proteins encoded by SLC3A1 and SLC7A9 associate to form the rBAT/b (0,+) amino acid transporter that is required for the reabsorption of cystine and dibasic amino acids in the proximal renal tubule and in the small intestine.22, 34, 35
In many cases, the diagnosis of cystinuria is made postnatally following recurrent urolithiasis in childhood. However, a HEC can be detected antenatally in a subset of patients before 36 weeks of gestation, with a high positive predictive value.19-21, 34 Cystine crystals are excreted with fetal urine in the amniotic fluid and are swallowed by the fetus. The defective reabsorption of cystine in the small intestine leads to an accumulation of cystine in the colon that appears hyperechogenic.21 However, the reason why the HEC sign is only present in some fetuses with cystinuria remains unknown. In cases of HEC, most mutations are located in the SLC3A1 gene, while in postnatal cases, both SLC3A1 and SLC7A9 can be mutated.21, 36
Cystinuria is an incurable disease, and recurrent cystine stones cause significant morbidity, with a high risk of renal failure. Management is based on dietary measures aimed at reducing cystine-saturated urine to avoid stones. This requires a low amino acids diet such as methionine and is largely consistent with a vegan diet. It is also important to maintain a high fluid intake to reduce cystine concentration and avoid cystine crystallization. Urine alkalinization drugs such as potassium citrate, potassium bicarbonate, and sodium bicarbonate should be offered to patients with recurrent cystine stones to reach cystine solubility pH, with a target urine pH of 7.0–7.5. If treatments with fluids, diet and alkalinization drugs is unsuccessful, cystine-binding thiol drugs (CBTD) as tiopronin and D-penicillamine should be proposed, with the aim of forming a soluble drug cystine complex. The ideal dose of CBTD is difficult to assess on the basis of urinary cystine concentration, determined by a capacity assay. The assay cannot distinguish between cystine and cystine–thiol drug complex, making the measurement of cystine concentration inaccurate. However, it is recommended to prescribe 1 mg of tiopronin for every 1 mg of cystine excreted per 24 h.3-37 In case of renal calculi, treatment is mainly symptomatic, with the use of analgesics. If treatment fails, ureteroscopy or extracorporeal shock wave lithotripsy may be considered.
The differential diagnosis of hyperechogenic kidneys is wide, which render antenatal counseling challenging as clinical presentation range from asymptomatic to early end-stage renal insufficiency. We report the first prenatal case in which isolated hyperechogenic kidneys are the only sonographic sign of congenital cystinuria: a diagnosis that should therefore be considered in such a context. Hyperechogenic colonic content is classically described as the leading feature of such a condition.16, 17 Additional cases and data are still required to better understand this atypical prenatal presentation.
AUTHOR CONTRIBUTIONS
Osaretin Pamela Aigbogun: Writing – original draft; writing – review and editing. Noémie Vancoppenolle: Writing – original draft. Sandra Coppens: Data curation; formal analysis. Martina Marangoni: Data curation. Elodie Elsen: Supervision; writing – review and editing. Marie Cassart: Supervision; validation. Caroline Gounongbe: Supervision; validation.
ACKNOWLEDGMENTS
The authors would like to thank the teams of gynecologists and pediatricians who collaborated on this clinical case.
FUNDING INFORMATION
No fund was allocated for this study.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
This study has been approved by the ethics committee of CHU Saint Pierre.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




