Coexistence of anti-MDA5 and anti-PL-7 in a patient with dermatomyositis: A case report
Abstract
Coexisting anti-MDA5 and anti-PL-7 antibodies are extremely rare. Anti-MDA5 is associated with rapidly progressive interstitial lung disease (RP-ILD), while anti-PL-7 is often associated with chronic or subacute ILD and better outcomes than RP-ILD. We report a 41-year-old woman diagnosed with dermatomyositis (DM)-associated ILD positive for anti-MDA5 and anti-PL-7.
1 INTRODUCTION
Dermatomyositis (DM) is an idiopathic systemic inflammatory myopathy with characteristic cutaneous findings, highly frequent pulmonary involvement, and typical autoantibodies, known as myositis-specific autoantibodies (MSAs). Myositis-specific autoantibodies comprise two groups: anti-aminoacyl-tRNA synthetase (anti-ARSs) and non-anti-ARSs. Each MSA type appears to cause a distinct clinical subtype.1, 2 Antimelanoma differentiation-associated gene 5 (anti-MDA5) is a non-anti-ARS, and the corresponding disease uniquely presents as DM-specific rashes, amyopathic muscle involvement, and rapidly progressive interstitial lung disease (RP-ILD) leading to a high 6-month mortality.3 Antithreonyl-tRNA synthetase (anti-PL-7) belongs to the anti-ARS group, accounting for 3%–5% of DM cases with chronic or subacute onset ILD and respond well to glucocorticoid (GC) treatment, but with frequent recurrence. It was reported that the rate of poor response to treatment was 30% in patients with anti-PL-7-positive DM.4-6 As for anti-MDA5-positive DM, RP-ILD is refractory to treatment, and the 6-month survival rate in Asian patients undergoing conventional immunosuppressant treatment is 28%–66%.7 The coexistence of anti-MDA5 and anti-PL-7 antibodies is extremely rare. Three DM cases were previously reported with coexisting anti-MDA5 and anti-ARS. One patient died due to RP-ILD.8-10
We report a case of DM-associated ILD with double positivity for anti-MDA5 and anti-PL-7. The patient had an effective response to treatment with GCs combined with a conventional immunosuppressor.
2 CASE PRESENTATION
A 41-year-old woman presented with productive cough and dyspnea for one month, accompanied by muscle pain in the extremities. She initially underwent antibiotic treatment in the local hospital for eight days and was transferred to our hospital due to symptom deterioration. The patient presented with Gottron papules in the elbows, facial erythema, and “mechanic's hands.” There was no history of exposure to communicable diseases.
3 INVESTIGATIONS
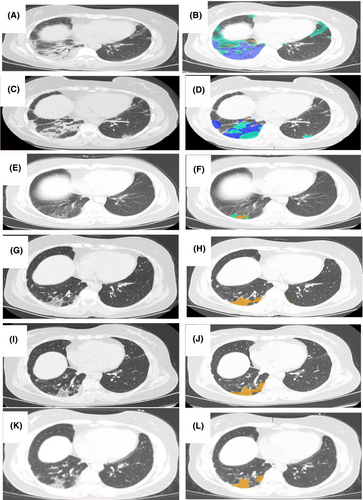
Laboratory tests showed a decreased PaO2/FiO2 ratio (342; normal values > 400). The anti-MDA5 and anti-PL-7 antibodies were both strongly positive, as measured by a commercial line blot immunoassay (Oumeng V Medical Laboratory, Hangzhou, China). Pulmonary function tests (PFT) showed that the percentage of predicted forced vital capacity (FVC%pred) was 65%, and the percentage of predicted diffusing lung capacity for carbon monoxide (DLCO%pred) was 80%. Electromyography was normal. Chest computed tomography (CT) revealed predominantly patchy ground-glass opacities (GGOs), peribronchovascular consolidation in the inferior right pulmonary lobe, and a small amount of pericardial fluid. Most lesions were localized, with irregular, ill-defined margins (Figure 1A). Therefore, we performed a computer-based quantitative CT evaluation with AVIEW software (Yizhiyuan Health Technology Co. Ltd, Zhejiang, China) to determine the percentage of parenchyma affected by the total lesions, GGOs, consolidation, and reticular pattern. These quantitative CT data were expressed as CT scores for each type of lesion. In ILD patients, quantitative CT scores of over time changes can provide an assessment of the treatment efficacy.11, 12 In addition, we used a previously validated ILD-GAP model (a single mortality prediction model for chronic ILD named after the variables including ILD subtype, gender, age, and pulmonary physiology)13 to identify the stage of disease severity and predict mortality. The ILD-GAP index was 0 (stage I). The patient was diagnosed with DM-associated ILD (double-positive MSAs: anti-MDA5 and anti-PL-7) as she met the Bohan and Peter criteria for DM.14
4 TREATMENT
The initial therapy comprised GCs (intravenous methylprednisolone [MPS] 40 mg/day [0.8 mg/kg/day] for five consecutive days) in combination with a conventional immunosuppressor (intravenous cyclophosphamide [IVCY] 400 mg [8 mg/kg] every two weeks). Intravenous immunoglobulins (IVIg, 2 g/kg/day for five consecutive days) was added as adjuvant therapy. The cough, dyspnea, and erythematous rashes were gradually resolved. The patient was discharged on Day 13 post the admission in our hospital, and was prescribed a maintenance therapy of oral MPS 40 mg/day and IVCY 400 mg every two weeks.
5 OUTCOME AND FOLLOW-UP
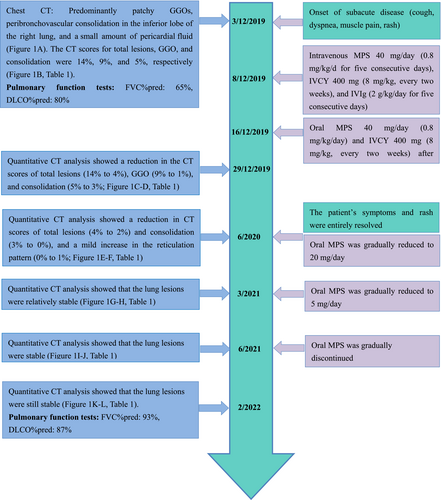
High-resolution CT (HRCT) was conducted at 27 days and at 7, 16, 19, and 27 months after the first admission and the quantitative CT scores for total lesions, GGOs, consolidation, and reticular pattern over time are analyzed (Figure 1, Table 1). Between the visits at 27 days and 7 months, the total lesions, GGOs, and consolidation identified on chest CT images progressively improved (Figure 1C–F, Table 1). After 7 months, the lesion absorption was more obvious, and a mild improvement in the reticulation pattern in the affected lung parenchyma was evident, demonstrating a strong response to treatment. The patient's symptoms and rash were entirely resolved. The oral MPS was gradually reduced to 20 mg/day. At the 16-, 19-, and 27-month hospital visits, the lung lesions on HRCT were stable and without relapse (Figure 1G–L, Table 1). Therefore, the oral MPS was further reduced to 5 mg/day and gradually discontinued. Pulmonary function tests were conducted at 27 months, showing that the percentage of predicted FVC was 93%, and the percentage of predicted DLCO was 87%. A time line of the patient's symptoms, radiographic images, pulmonary function tests, and treatment history is shown in Figure 2.
| Quantitative CT scores | Baseline | 26 days | 7 months | 16 months | 19 months | 27 months |
|---|---|---|---|---|---|---|
| Total lesions (%) | 14 | 4 | 2 | 1 | 1 | 1 |
| GGO (%) | 9 | 1 | 1 | 0 | 0 | 0 |
| Consolidation (%) | 5 | 3 | 0 | 0 | 0 | 0 |
| Reticular pattern (%) | 0 | 0 | 1 | 1 | 1 | 1 |
- Abbreviations: CT, computed tomography; GGO, ground-glass opacity.
6 DISCUSSION
Different MSA-positive DM subgroups are associated with different clinical features.1 Several studies reported that anti-MDA5 is strongly associated with RP-ILD and poor survival in patients with DM-ILD, particularly in Asian populations.3, 15 Muscle involvement in patients with anti-MDA5-positive DM is generally mild. The dominant CT findings in patients with anti-MDA5 ILD are mainly inferior peripheral and peribronchovascular consolidation/GGO or random GGO pattern without intralobular reticular opacities, indicating organized pneumonia (OP) or localized diffuse alveolar damage (DAD). Anti-MDA5-ILD patients usually experience an acute onset, RP-ILD course, and high mortality rate.1, 16 Anti-PL-7 is a rare subtype of anti-ARSs, causing predominantly nonspecific interstitial pneumonia, as seen on HRCT and pathology examinations. In a previous study of 18 cases of anti-PL-7-positive DM,17 the median age at diagnosis was 52.5 years, most patients were women (83%), and the most common manifestations were ILD (77%), myositis (75%), arthritis (56%), and pericardial effusion (50%). In a group of Japanese patients with myositis, anti-PL-7 was associated with lower levels of lactate dehydrogenase and creatine kinase, milder muscle weakness, and higher frequency of pericardial effusion than anti-Jo1-positive DM.18 Patients positive for anti-ARSs usually present with chronic or subacute onset, and the characteristic CT findings in patients with anti-ARS-ILD are GGO and reticulation, predominantly distributed in the inferior lobes and subpleural regions.19
In 2017, Naniwa et al.8 first reported a case of double anti-MDA5/anti-PL-7-positive DM-ILD in a 43-year-old female Japanese. This patient initially experienced a rapidly deteriorating ILD, characterized by patchy consolidation, GGO, subpleural line, and reticular opacities in the right lung lobe. The disease was partially controlled after a triple treatment (GCs, calcineurin inhibitor [CNI], and IVCY) and adjuvant therapy. At the 22-month follow-up, chest CT showed progression of traction bronchiectasis and bronchioloectasis in the affected lung parenchyma. The disease course and imaging findings were considered a combination of features of anti-ARS and anti-MDA5 ILD. In 2018, Takeuchi et al.9 reported the case of a 53-year-old Japanese woman with clinically amyopathic DM, double-positive for anti-MDA5 and anti-EJ. The CT patterns were characterized by lower GGOs, reticulation, and bilateral traction bronchiectasis at disease onset. The patient responded to an initial treatment with GCs and CNI, and later experienced repeated recurrences and aggravation of the disease. The initial clinical course was consistent with anti-ARS ILD. The occurrence of sudden acute exacerbation of ILD, refractory to high-dose GCs and immunosuppressants, was possibly associated with the presence of anti-MDA5. In 2020, Li et al.10 reported the case of a 27-year-old Hispanic DM patient with coexisting anti-MDA-5 and anti-PL-7, who died due to severe respiratory failure secondary to RP-ILD despite maximal supportive treatments were provided for 33 days. The disease course was consistent with anti-MDA5 ILD.
In this study, we reported a rare case of DM-ILD and coexisting anti-MDA5 and anti-PL-7 in a Chinese patient. Compared with the three previously reported cases of double-positive anti-ARS and anti-MDA5 DM-ILD, the HRCT features in this patient were predominantly patchy GGO and peribronchovascular consolidation in the inferior lobes and subpleural regions, without intralobular reticular opacities upon admission. These findings most likely indicated OP or localized DAD and a subacute-to-acute course, in line with anti-MDA5-positive cases. During the follow-up period, HRCT revealed marked improvement of the GGO and consolidation, and a mild reticulation pattern was seen in the affected lung parenchyma, which is a clinical course more often associated with anti-ARS ILD. Pericardial effusion was also observed in our patient, usually linked to anti-PL-7. In this case, the patient had no muscle involvement, consistent with previous studies shown that patients with anti-MDA5 DM had absent or minimal muscle involvement. This presentation was likely associated with anti-MDA5 reactivity in the skeletal muscle inflammation pathway. Regarding skin involvement, the presentation was consistent with the findings reported by Hamaguchi et al.20 predominantly observed in DM patients with positive anti-ARS, without the rash uniquely related to anti-MDA5. A detailed comparison of the four cases is presented in Table 2.
| Case 1 | Case 2 | Case 3 | Our case | |
|---|---|---|---|---|
| Authors | Naniwa et al. | Takeuchi et al. | Li et al. | NA |
| Reference | 12 | 13 | 14 | NA |
| Year | 2017 | 2018 | 2020 | 2022 |
| Ethnicity | Japanese | Japanese | Hispanic | Chinese |
| Age (years)/sex | 43/F | 53/F | 27/F | 41/F |
| Double-positive MSAs | Anti-MDA5 and anti-PL-7 | Anti-MDA5 and anti-EJ | Anti-MDA5 and anti-PL-7 | Anti-MDA5 and anti-PL-7 |
| Skin manifestations | Gottron's papules, heliotrope rash, facial erythema, and shawl sign | Gottron papules, heliotrope rash, facial erythema, mechanic's hands, and periungual erythema | Gottron's patches | Gottron papules, facial erythema, and mechanic's hands |
| Myositis | Yes | Yes | No | No |
| Initial CT features | Patchy consolidations and GGO, subpleural line, and reticular opacities | Reticulation and GGO | GGO | Predominantly GGO and patchy peribronchovascular consolidation |
| Predominantly involved pulmonary lobe | Inferior right lobe | Bilateral, predominantly distributed in the inferior lobes | Bilateral, whole lungs | Inferior right lobe |
| Predominant distribution | Peripheral | Peripheral | Diffuse | Diffuse |
| Follow-up CT features | Progression of traction bronchiectasis in affected lung parenchyma | Newly developed random GGO | NA | Decreased GGO consolidation, and pericardial fluid |
| Pericardial effusion | No | No | No | Yes |
| ILD form | Acute/subacute | Acute/subacute | Acute/subacute | Acute/subacute |
| RP-ILD | Yes | No | Yes | No |
| Clinic relapse | Yes | Yes | NA | No |
| Initial treatment | GCs, CNI, and IVCY | GCs and CNI | GCs, IVIg, and rituximab | GCs, IVCY, and IVIg |
| Outcome | Worsened | Worsened | Death | Improved |
| Cause of death | NA | NA | Respiratory failure | NA |
- Abbreviations: Anti-MDA5, anti-melanoma differentiation-associated gene 5; anti-PL-7, anti-threonyl-tRNA synthetase; CNI, calcineurin inhibitor; CT, computed tomography; GCs, glucocorticoids; GGO, ground-glass opacities; ILD, interstitial lung disease; IVCY, intravenous cyclophosphamide; IVIg, intravenous immunoglobulins; MSAs, myositis-specific autoantibodies; NA, not applicable; RP-ILD, rapidly progressing interstitial lung disease.
GCs are the first line of therapy in DM. Moreover, the early initiation of combined immunosuppressive therapy is an evidence-based recommendation for treating rapidly progressing anti-MDA5-positive DM-ILD, as reported by Japanese colleagues.21 Upon the first hospital admission, this patient was in the early phase of the disease, according to the ILD-GAP index. The symptoms and lung lesions seen on HRCT improved with treatment, and the patient had no relapse for over 27 months under combined anti-inflammatory and immunosuppressive therapy. It is important to obtain an early diagnosis and promptly start a combined treatment for anti-MDA5-positive DM-ILD to transform it from a rapidly progressive form to a chronic disease.16 In addition, the ILD-GAP staging system could help clinicians to predict mortality more accurately in patients with DM-ILD.13
7 CONCLUSION
The coexistence of anti-MDA5 and anti-PL-7 is extremely rare in DM patients. Our report showed that the clinical features of this case correlate closely with the DM course associated with both anti-MDA5 and anti-PL-7 circulating antibodies. The patient had a remarkable response to GCs combined with a conventional immunosuppressor. This case suggests that early recognition and prompt treatment of DM-ILD are crucial to avoid the development of RP-ILD. Close follow-up could help determine whether the early therapeutic modalities are sufficiently effective. Further studies exploring the possible etiology and pathogenetic mechanisms of this mysterious disease will be helpful to screen, evaluate, and manage appropriately the patients affected.
AUTHOR CONTRIBUTIONS
Hongyan Fu: Writing – original draft; writing – review and editing. Shaofeng Sun: Formal analysis. Hong Zhang: Formal analysis. Shuhong Chi: Visualization. Weirong Ma: Visualization. Guilan Yang: Visualization. juan chen: Funding acquisition; writing – original draft; writing – review and editing.
ACKNOWLEDGMENT
We thank Dr. Zhengping Zhang of the Department of Radiology at the General Hospital of Ningxia Medical University for his contribution in the chest CT image interpretation.
FUNDING INFORMATION
This work was supported by grants from the Natural Science Foundation of Ningxia Hui Autonomous Region (No. 2020AAC02001), Key Research and Development Program of Ningxia Hui Autonomous Region (No. 2018BEG03035), and Ningxia Medical University Foundation (A project to construct first-class disciplines).
CONFLICT OF INTEREST
The authors declare no competing interests.
ETHICAL APPROVAL
This study was approved by the Ethics Committee and Institutional Review Board of the General Hospital at Ningxia Medical University, Yinchuan, China (2022-32). The patient provided informed consent for publication.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.