Liver transplantation following late-onset hepatic sinusoidal obstruction syndrome occurred beyond 1-year postallogeneic hematopoietic stem cell transplantation
Abstract
Late-onset HOVD should be taken into consideration when patients develop liver dysfunction and/or weight gain no matter how long post-HSCT. Solid organ transplantation offers a valuable therapeutic option for selected patients with single organ failure after HSCT without adverse impact on graft function or overall outcomes.
1 INTRODUCTION
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (HVOD/SOS) is a rare but lethal complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). For decades, the diagnosis of HVOD is primarily based on clinical symptoms including weight gain with ascites, painful hepatomegaly, and jaundice according to the Seattle and Baltimore criteria. Although many studies of HVOD have been reported, the incidence data were varying because of the small sample size, variations in the conditioning regimen, and different diagnostic criteria.1 The European Society for Blood and Marrow Transplantation (EBMT) proposed the revised diagnosis and severity criteria of VOD/SOS in 2016, aimed at overcoming the lack of specificity and sensitivity of the previous criteria (Table S1).2 Generally, HVOD occurs within 21 days post-HSCT, but in 15%–20% HVOD can develop later and few cases of late-onset HVOD have been reported. For now, the late-onset cases of HOVD reported are extremely rare and almost occurred within 3 months after allo-HSCT.3-5 Here, we presented a case of liver transplantation following late-onset HVOD occurred more than 1 year after allo-HSCT for the first time.
2 CASE PRESENTATION
A 48-year-old woman was diagnosed with myelodysplastic syndrome-refractory anemia with excess blasts type 2 (MDS-EBII) in November 2017 (Figure S1). She achieved complete remission (CR) after two chemotherapy courses with decitabine combined with CAG regimen (cytarabine 10 mg/m2 q12h, days 1–14; aclarubicin 14 mg/m2 qd, Days 1–4; granulocyte colony-stimulating factor 200ug/m2 qd, Days 1–14; (Figure S2). Her elder brother was identified as her HLA-matched sibling donor with major ABO mismatch (recipient O+, donor B+). This patient received allo-HSCT in March 2018. Conditioning therapy consisted of decitabine 25 mg/d intravenously for 5 consecutive days on Days -13d to -9d; cytarabine 4 g/m2/d intravenously on Days −10 to −9; busulfan 3.2 mg/kg/d once daily on Days −9 to −6; cyclophosphamide 1.8 g/m2/d administered on Days −5 to −4; and p-ALG® (Wuhan Institute of Biological Products, China) 25 mg/kg/d intravenously for 4 consecutive days, on Days −5 to −2. Prophylaxis for graft versus host disease (GVHD) consisted of cyclosporine A (CsA), mycophenolate mofetil (MMF), and short course of methotrexate (MTX). The patient received intravenous prostaglandin E1 (PGE1) 0.3 μg/kg/h infusion continuously from the day before the start of conditioning to 21 days after HSCT for VOD prevention. The CD34 and mononuclear count (MNC) of stem cells infused were 3.36 × 106/kg and 21 × 108/kg, respectively. An absolute neutrophil count higher than 0.5 × 109/L and a platelet count higher than 20 × 109/L were achieved on Day +12. The chimerism of CD3+ T cell was 96.5% at the third month after allo-HSCT. Acute GVHD (aGVHD) was not observed. The blood type of the recipient did not convert to the donor's blood type at the end of the third month after allo-HSCT. The patient became transfusion-dependent due to anemia. Donor lymphocyte infusion (DLI) was performed 4 months after allo-HSCT. The chimerism of CD3+ T cell was 100% 2 weeks after DLI, and GVHD was not observed. But, conversion of the blood type was still incomplete. Thus, prednisone 20 mg per day and sirolimus 1 mg per day were taken. Finally, 9 months after allo-HSCT, the blood type of this patient completely converted to donor's blood type. And 3 months later, on March 2019, all drugs including immunosuppressant drugs and anti-infective agents were stopped.
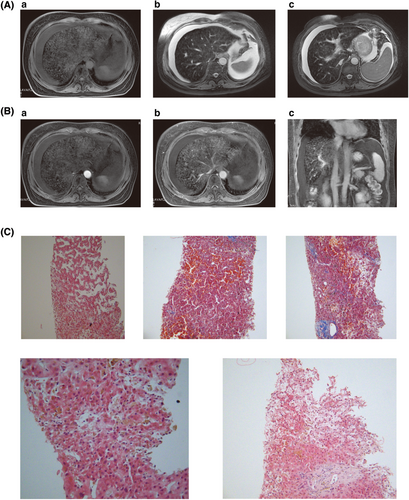
On July 2019, 16 months after allo-HSCT, a mild elevation in transaminase and total serum bilirubin appeared. The tests for viral hepatitis, autoimmune hepatitis, and infective causes came back negative. The direct Coombs test, the levels of free hemoglobin and haptoglobin, the coagulation function, and the result of abdominal ultrasonography were all normal. Ultrasonography showed normal liver parenchyma, regular biliary tract, and normal-sized spleen with ascites. The concentration of ferritin in peripheral blood was 24693ug/L. The expression of biomarker for aGVHD including sST2, REG3a, sTNFR1, IL-6, and IL-8 was significantly increased. The abdominal paracentesis was performed and showed presence of exudate fluid with many cells (neutrophil 5%, lymphocyte 65%, and macrophage 30%). Thus, the diagnoses of hepatic chronic GVHD (cGVHD) and iron overload were made. Consequently, deferasirox and treatments against cGVHD including methylprednisolone, tacrolimus, azathioprine, ruxolitinib, rituximab, ibrutinib, infusion of mesenchymal stem cells, and plasma exchange (PE) were performed (The details of treatment course are shown in Figure 1). During the treatment, the concentration of ferritin was declined gradually. But, the levels of transaminase and total serum bilirubin further increased. Decreased vision, massive ascites, abdominal pain, crura edema, oliguria, and weight gain(>10%) were emerged. Liver magnetic resonance imaging (MRI) showed heterogeneous signal of liver parenchyma in T1WI and T2WI, hepatic vein, and hepatic segmental interior vena cava narrowing, periportal edema, and ascites. After administration of the contrast agent, during the portal phase, a diffuse heterogeneity in hepatic enhancement was observed (Figure 2A,B). Therefore, late-onset HVOD was taken into consideration and hepatic transjugular biopsy was performed. According to histology results, no infiltration of inflammation cells in the portal area of liver was found, and immunological injuries of graft versus host reaction such as interlobular bile duct epitheliitis and vascular endothelitis were not observed, suggesting the exclusion of hepatic GVHD. The results of histology also showed partial dilatation and sinusoidal engorgement and extravasation of red blood cells through the space of Disse, associated with perivenular hepatocyte necrosis (at liver inflammation stages of G0, at liver fibrosis stages of S0) indicating pathological changes in the early stage of HVOD (Figure 2C, Table S2).6 This patient was finally diagnosed very severe HVOD by the new EBMT criteria.2
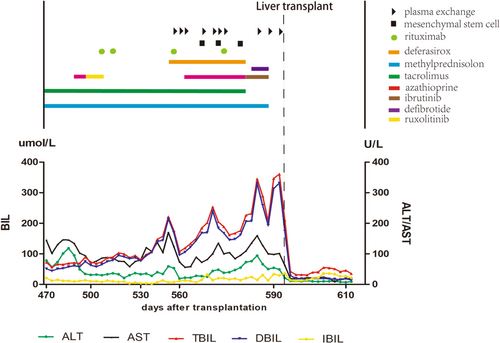
Intravenous defibrotide was used at the dose of 6.25 mg/kg QID for 7 days along with appropriate supportive care. Unfortunately, the liver dysfunction continued to deteriorate and anuria appeared. Therefore, a suggestion of liver transplant was proposed to the patient. On November 4, 2019, a liver from unrelated donor was successfully transplanted. Methylprednisolone, tacrolimus, and MMF were used for prophylaxis of transplant rejection under the guidance of doctors. Liver function almost entirely returned to normal when the patient got out of the hospital. After 2-year follow-up, the patient recovered well.
3 DISCUSSION
Liver disease occurs in up to 80% of patients following HSCT ranging from mild and reversible elevation of serum transaminases, to fatal hepatic failure. HVOD is a rare and highly lethal condition of the liver after HSCT. It is associated with various risk factors (Table S1C). Several risk factors existed in this patient including non-T-cell-depleted transplant, myeloablative-conditioning regimen, high-dose busulfan-based regimen, hepatotoxic drugs, and iron overload, which led to a higher risk of developing HVOD. According to EBMT criteria, late-onset HOVD was defined as those occurred beyond Day 21 after HSCT.2 But, the time of onset in majority of cases was less than 100 days after HSCT. The clinical signs of this patient appeared beyond 16 months post-HSCT and 12 months after DLI. The patient did not experience either painful hepatomegaly or weight gain, and only a mild elevation in transaminase and total serum bilirubin level in the early stage. Given that liver dysfunction after HSCT could be caused by many reasons such as hepatic GVHD, drug toxicity, viral infection, iron overload, and sepsis, cGVHD rather than HVOD was regarded as the main reason for liver dysfunction of this patient based on patient history and various laboratory testing. Nevertheless, the treatments against cGVHD were ineffective and the typical clinical symptoms of HVOD were observed during treatments. At present, no specific laboratory biomarker is available so that imaging techniques and liver biopsies become very important to differential diagnosis.7, 8 Imaging techniques including doppler ultrasound (US), abdominal computed tomographic (CT) scan, MRI, and 18F-fluorodeoxyglucose positron emission tomography/CT (PET/CT) have become the first-line methods in differentiating HVOD from GVHD. The experts agreed that T2-weighted MRI can indicate the degree of hepatic siderosis and will be useful in determining the need for VOD/SOS prophylaxis.9 In the literature, MRI scans of HVOD showed hepatomegaly, ascites, hepatic vein narrowing, gallbladder wall thickening, periportal cuffing, or patchy signal enhancement of the liver.10-12 The MRI of this patient presented similar imaging findings suggesting the possibility of HVOD. Histological evidence of HVOD remains the gold standard (but not mandatory) for the diagnosis. HVOD may exhibit acute, subacute, and chronic features, depending upon when liver material is obtained. In the early stages, the histological features of HVOD consist of dilation of sinusoids with engorgement due to red blood cells escaping through the space of Disse. At a later stage of HVOD, liver biopsy reveals incomplete to complete fibrous obliteration of the lumina of central venules with centrilobular area fibrosis.9, 13 In this case, the tissue of liver biopsy showed histological changes in the early stage of HVOD. Finally, the definite diagnosis of very severe late-onset HVOD was made.
The British Committee for Standards in Haematology (BCSH) and the British Society for Blood and Marrow Transplantation (BSBMT) (BCSH/BSBMT) guideline recommends defibrotide for the treatment of HVOD in adults and children.14-18 The recommended dose of defibrotide was 25 mg/kg per day, and the treatment should be continued until resolution of symptoms, or for a minimum of 21 days.15 In this case, defibrotide was used immediately after HVOD was diagnosis. Unfortunately, no sign of improvement was shown after 7 days of treatment of defibrotide, and rapid development was observed in present case. Therefore, a suggestion of liver transplantation before the development of multiple organ dysfunction (MOD) was given.
HSCT recipients with end-stage organ dysfunction generally have very poor outcomes, and solid organ transplantation (SOT) may offer a viable therapeutic option for selected patients.18-20 Liver failure in HSCT recipients can be related to infectious hepatitis, HVOD, GVHD, sepsis, or hemosiderosis. According to the literature, the most common indications for liver transplantation are HVOD within the first 100 days and GVHD after 100 days post-HSCT [29–31]. Studies showed that previous HSCT does not seem to have an adverse impact on graft function or overall outcomes after SOT. The rate of SOT failure was not markedly different in SOT recipients with or without GVHD at time of SOT, suggesting that GVHD does not necessarily contribute to a worse outcome.18-20 EBMT reported that survival rates for liver graft recipients at 5 years post-HSCT were 71%.18 The outcomes of liver transplant in recipients who receive a liver transplant 6–12 months after HSCT showed better results. And, liver transplantation had a higher rate of graft rejection compared with renal transplantation.19 In this case, the patient performed liver transplantation with a unrelated liver graft at 19 months post-HSCT. After 3-year follow-up, the relapse of MDS, transplant rejection, and liver dysfunction were not observed.
4 CONCLUSION
Although late-onset HVOD mainly occurred within 100 days after HSCT, it must be taken into consideration when patients develop liver dysfunction and/or weight gain no matter how long post-HSCT. Imaging techniques and liver biopsies are very useful tools in differential diagnosis when the clinical criteria for HVOD are not entirely fulfilled.
AUTHOR CONTRIBUTIONS
Jin Yin put all data together and wrote the manuscript. Hui Guo gave the pathologic interpretation. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable.
FUNDING INFORMATION
This study was supported by two grants from the National Natural Science Foundation of China (81700174).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
DATA AVAILABILITY STTATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ETHICAL APPROVAL
The study was approved by the Medical Ethics Committee of the hospital. The study was performed according to the Declaration of Helsinki.
CONSENT
Consent from the patient is obtained. Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.