A case of shock after STEMI: Think beyond the cardiogenic one
Abstract
Acute ST-segment elevation myocardial infarction (STEMI) can typically complicate with the development of cardiogenic shock; nevertheless, other less frequent types of shock may occur, including adrenal crisis (AC). We describe a case of STEMI complicated by AC and, for the first time, AC-induced focal takotsubo syndrome.
1 INTRODUCTION
Dual antiplatelet therapy (DAPT) and percutaneous coronary intervention (PCI) are indicated for the treatment of acute ST-segment elevation myocardial infarction (STEMI),1 nevertheless STEMI can complicate with the development of shock.
In this context, the most frequent shocks are the cardiogenic and the hypovolemic ones. The former may be a consequence of mechanical, allergic (Kounis syndrome), procedural and arrhythmic complications due to both the STEMI itself and/or its treatment and the administration of contrast medium while performing the coronary angiography (CA).1-6 By contrast, the latter is typically a consequence of a major bleeding due to the administration of antiplatelet and anticoagulants agents. Nevertheless, rarer causes than those described above may sometimes occur leading to shock. These ones include infections and the development of adrenal insufficiency, resulting in septic shock and adrenal crisis (AC),7 respectively. The latter, in turn, may sometimes lead to the development of Takotsubo syndrome (TTS),8 resulting in AC-induced TTS.
Because all of these conditions are burdened with high early mortality and most of them may present with ST-segment elevation (STE) associated with left ventricle ejection fraction (LVEF) reduction and regional wall motion abnormalities (RWMA), it is critical to make the correct diagnosis to initiate appropriate treatment and reduce mortality.
Here we report the case of a woman with left anterior descending (LAD) coronary artery occlusion-related STEMI treated with PCI and complicated by the development of AC and, for the first time, AC-induced focal takotsubo syndrome (fTTS).
2 CASE PRESENTATION
A 74-year-old woman in good clinical condition, presented to the emergency department with acute anterior STEMI (Killip class I–II), normal blood pressure and LVEF of 35% with apical akinesia and mid-apical septal hypokinesia on transthoracic echocardiogram (TTE; Figure 1, Table 1, Video S1a). Except for the recent diagnosis of polymyalgia rheumatica and the ongoing STEMI, the patient had no other previous disease, cardiovascular risk factors, allergies, recent infections and was not taking any medications. Nevertheless, a short course of low-dose prednisone (7.5 mg/day) was discontinued 36 h before admission.
| Reference values | Emergency Department | ICCU admission (1 h after PCI) | Shock (7 h after PCI) | Refractory shock (10 h after PCI) | 2 h after hydrocortisone administration | 2nd day (18 h after hydrocortisone administration) | 3rd day | Discharge | |
|---|---|---|---|---|---|---|---|---|---|
| Vital signs | |||||||||
| Systolic blood pressure (mm Hg) | >90 | 110 | 105 | 68 | 67 | 90 | 115 | 118 | 112 |
| Diastolic blood pressure (mm Hg) | >60 | 60 | 62 | 40 | 41 | 52 | 60 | 68 | 65 |
| Peripheral oxygen saturation (%) | >90 | 94 | 96 | 92 | 92 | 93 | 94 | 94 | 96 |
| Breath rate (breaths per minute) | 14–20 | 22 | 20 | 32 | 32 | 26 | 24 | 24 | 18 |
| Pulse rate (beats per minute) | 60–100 | 95 | 86 | 110 | 108 | 100 | 98 | 95 | 74 |
| Maximum Temperature (°C) | 36.0–37.9 | 36.2 | 37.1 | 36.8 | 36.7 | / | 36.4 | 36.3 | 35.7 |
| Arterial blood gas analysis | |||||||||
| PaO2/FiO2 (mm Hg) | >300 | 365 | 354 | 336 | 329 | 384 | 430 | 436 | / |
| PaCO2 (mm Hg) | 36–44 | 33 | 36 | 26 | 25 | 30 | 32 | 32 | / |
| pH | 7.36–7.44 | 7.45 | 7.40 | 7.52 | 7.48 | 7.48 | 7.45 | 7.44 | / |
| Bicarbonate (mmol/L) | 22–26 | 22 | 23 | 18 | 19 | 21 | 22 | 22 | / |
| Lactate (mmol/L) | 0.5–1.5 | 1.5 | 1.4 | 2.0 | 2.1 | 1.7 | 1.4 | 1.2 | / |
| Echocardiographic findings | |||||||||
|
LVEF (%) (Simpson's biplane method) |
54–74 (American Society of Echocardiography) | 35 | 35 | 30 | 30 | 35 | 40 | 40 | 45 |
| Hemodynamic values | |||||||||
| Cardiac index (L/min/m2) | 2.3–4.2 | / | / | 2.7 | 2.6 | 3.0 | 3.3 | 3.5 | / |
| Mean arterial pressure (mm Hg) | > 65 | / | 77 | 45 | 46 | 68 | 80 | 86 | / |
| Central venous pressure (mm Hg) | 8–12 | / | / | 6 | 6 | 8 | 9 | 10 | / |
| Central venous oxygen saturation (%) | 70–80 | / | / | 67 | 68 | 70 | 73 | 73 | / |
|
Systemic vascular resistances (dyn × s/cm5) |
900–1400 | / | / | 681 | 711 | 833 | 924 | 1037 | / |
| Laboratory exams | |||||||||
| White blood cells count (× 103/mcgl) | 4.0–10.8 | 11.2 | 11.4 | 13.2 | 13.1 | 13.6 | 16.2 | 15.6 | 10.2 |
| Absolute neutrophils count (× 103/mcgl) | 1.5–7.5 | 7.6 | 7.7 | 9.2 | 9.1 | 9.3 | 13.1 | 13.6 | 8.6 |
| Absolute eosinophils count (× 103/mcgl) | 0.1–0.5 | 0.2 | 0.2 | 0.3 | 0.3 | 0.5 | 0.4 | 0.3 | 0.2 |
| Hemoglobin (g/dl) | 12.0–16.0 | 11.8 | 11.4 | 10.5 | 9.8 | 9.3 | 10.3 | 10.9 | 11.7 |
| Platelets count (× 103/mcgl) | 130–424 | 287 | 269 | 258 | 267 | 301 | 331 | 289 | 324 |
| Creatinine (mg/dl) | 0.4–1.1 | 0.8 | 0.9 | 1.4 | 1.5 | 1.3 | 1.1 | 1.0 | 0.7 |
| eGFR (ml/min/1,73 m2) | >90 | 75 | 70 | 48 | 46 | 54 | 62 | 69 | 78 |
| Glucose (mg/dl) | 74–106 | 88 | 80 | 77 | 74 | 104 | 111 | 98 | 94 |
| Aspartate aminotransferase (U/L) | <43 | 346 | 324 | 339 | 321 | 295 | 202 | 166 | 33 |
| Alanine aminotransferase (U/L) | <45 | 231 | 224 | 245 | 214 | 201 | 188 | 147 | 39 |
| Gamma glutamyl transferase (U/L) | <38 | 40 | 42 | 53 | 54 | 48 | 42 | 38 | 32 |
| Total bilirubin (mg/dl) | <1.2 | 1.0 | 1.1 | 1.5 | / | 1.4 | 1.1 | 1.1 | 0,9 |
| Sodium (mmol/L) | 132–146 | 134 | 135 | 129 | 128 | 133 | 134 | 135 | 142 |
| Potassium (mmol/L) | 3.5–5.5 | 3.7 | 3.9 | 4.9 | 5.0 | 4.2 | 3.7 | 3.6 | 4.1 |
| C-Reactive protein (mg/L) | <5 | 7 | / | 27 | / | 34 | 32 | 29 | 2 |
| Procalcitonin (ng/ml) | <0.2 | / | / | 0.5 | / | 0.5 | 0.4 | 0.3 | 0.1 |
| hs-cTn I (pg/ml) | <37 | 8742 | 9423 | 10,182 | 10,987 | 10,269 | 8754 | 3621 | 123 |
| NT-proBNP (pg/ml) | <450 | 14,634 | 16,387 | 26,672 | 28,905 | 25,712 | 22,789 | 14,520 | 744 |
| International Normalized Ratio (INR) | 0.8–1.2 | 1.1 | 2.2 | 2.5 | 2.3 | 1.8 | 1.7 | 1.6 | 1.2 |
| Activated partial thromboplastin time (s) | 30–40 | 38 | 54 | 68 | 65 | 57 | 49 | 42 | 40 |
| Cortisol (mcg/dl) | >10–15 mcg/dl (during an acute illness)a | / | / | / | 7 | / | / | / | / |
| ACTH (pg/dl) | 5–20 | / | / | 794 | / | / | / | / | |
| Microbiology exams | |||||||||
| Blood coltures | / | / | / | Negatives | / | / | / | / | |
| Urine coltures | / | / | / | Negatives | / | ||||
- Abbreviations: ACTH, Adrenocorticotropic hormone; eGFR, estimated glomerular filtration rate; hs-cTn I: high sensitivity-cardiac Troponin I; ICCU, intensive cardiac care unit; LVEF, left ventricle ejection fraction; NT-proBNP, N-terminal pro-BNP; PaCO2, partial pressure of arterial carbon dioxide; PaO2/FiO2, arterial oxygen partial pressure to fractional inspired oxygen ratio.
- a Reference number.9
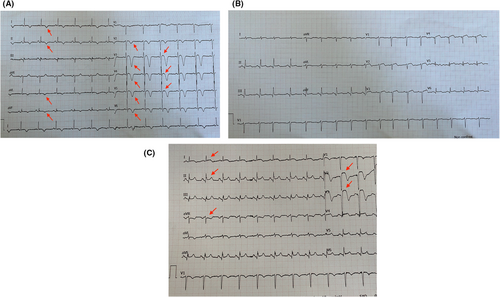
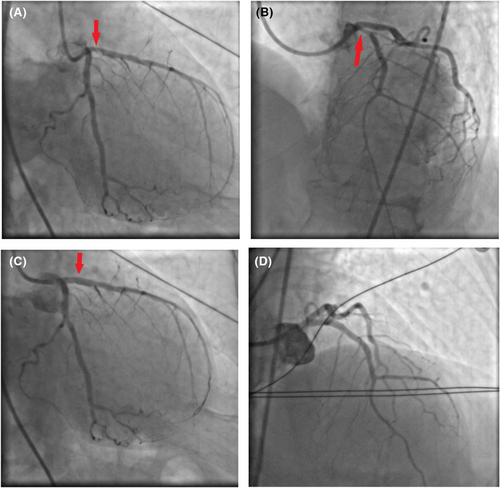
After the administration of acetylsalicylic acid, ticagrelor and unfractionated heparin, the right femoral artery was cannulated and CA was performed. Through PCI, a 12-mm- length zotarolimus-eluting stent was placed over the proximal tract of the LAD, where a critical occlusion was found (Figure 2A,B; Video S2a,b). Thrombolysis in myocardial infarction (TIMI)-III flow and regression of STE on electrocardiogram (ECG) were achieved (Figures 1B and 2C; Video S2c), then the patient was transferred to the intensive coronary care unit (ICCU; Table 1).
Seven hours later, the patient suddenly developed chest pain, arterial hypotension and shock (Table 1). Mental confusion, sweating, a mild systolic murmur and absence of fever, jugular venous distension, pulmonary congestion and femoral access-site complications were revealed by clinical evaluation. Sinus tachycardia associated with anterolateral STE and reciprocal depression on lead aVR were shown on ECG (Figure 1C). LVEF of 30%, mid-apical septal akinesia, small and collapsing inferior vena cava (Video S1b) and absence of acute valvular dysfunction, pericardial effusion and pleural B-lines were shown by TTE and pulmonary ultrasound, respectively. Accordingly, cardiogenic shock due to mechanical complications and arrhythmias were ruled out. Then, a central venous catheter was placed, crystalloids, norepinephrine and dobutamine were started, lab exams were performed and other causes of anterior STE-associated shock were investigated.
Firstly, both iatrogenic aortic dissection (IAD) and major bleeding resulting in hypovolemic shock due to femoral artery cannulation and administration of antiplatelet and anticoagulant agents were ruled out. Computed tomography (CT) scans of the head, chest and abdomen (extended to the proximal tract of the femoral arteries) as well as lab exams showed neither intimal tears along the aorta and any active source of bleeding nor significant drop in hemoglobin values, respectively (Table 1). Subsequently, intra-aortic balloon pump (IABP) was placed and cardiogenic shock due to both acute stent thrombosis (AST) and to a hypersensitivity coronary artery reaction resulting in Kounis syndrome (KS), was ruled out by performing again CA, that showed normal flow over all coronary arteries (Figure 2D; Video S2d).
Then, because mild anemia, hyponatremia, increased creatinine values and a slight increase of inflammatory biomarkers associated with low systemic vascular resistances were revealed by lab exams and hemodynamic monitoring (Table 1), septic shock (SS) was suspected. Accordingly, dobutamine was discontinued, norepinephrine was titrated up to 3.0 mcg/kg/min, microbiological exams were performed and broad-spectrum antibiotics were started. Despite these arrangements, a refractory shock associated with both LVEF reduction and a new regional akinesia was still ongoing 3 hours after clinical deterioration. In addition, after reviewing the CT images, a likely infectious focus was not detected. Based on all these findings, SS became unlikely as well.
Subsequently, given the recent discontinuation of prednisone as well as the occurrence of vasopressor-refractory shock during an acute illness, AC with AC-induced fTTS was suspected. Accordingly, plasma cortisol and adrenocorticotropic hormone were sampled and intravenous hydrocortisone (100 mg bolus, followed by infusion at 8.3 mg/h) was administered. Over the next 2 hours, hemodynamic stabilization, regression of symptoms and a slight increase of LVEF to 35% were achieved. In addition, regression of anterior STE and improvement of mid-apical septal motion, which became hypokinetic, were also obtained (Table 1). Over the next days, the patient gradually recovered. Blood pressure remained within the normal range, LVEF increased up to 45% associated with overall improvement of the regional kinesis (Video S1c), cardiac biomarkers decreased, all microbiological exams and inflammatory biomarkers were not suggestive of infection and cortisol values were typical of adrenal insufficiency9 (Table 1). IABP was removed, norepinephrine was tapered and then discontinued, antibiotics were stopped and hydrocortisone was tapered and switched to oral formulation. On day seven, the patient was discharged with conventional therapy for myocardial infarction, prednisone 25 mg/day and endocrinological evaluation was scheduled.
Our final diagnosis, strengthened by the exclusion of other causes of shock, was STEMI complicated by AC with AC-induced fTTS. Indeed, a few hours after hydrocortisone administration, STE-associated shock and symptoms resolved and global and regional left ventricular kinesis improved. Moreover, no further myocardial complications were detected during recovery. Specifically, sudden shock, symptoms, low systemic vascular resistance, cortisol values and other biochemical abnormalities were attributable to AC, whereas anterior STE, normal flow over all coronary arteries, deterioration of global and regional kinesis characterized by prompt recovery, and absence of further myocardial complications, could be explained by fTTS.
The graphical abstract summarizes the case described above.
3 DISCUSSION
After PCI-treated STEMI, both cardiologic and noncardiologic conditions may present with anterior STE-associated shock.
Cardiologic conditions are more likely and include cardiogenic and hypovolemic shock. Cardiogenic shock may be a consequence of mechanical, procedural, arrhythmic and allergic complications, while hypovolemic shock is typically a consequence of a major bleeding related to the administration of antiplatelet and anticoagulant agents. In this context, while myocardial free wall rupture, IAD and AST are recognized among mechanical and procedural complications, KS, a hypersensitivity coronary artery reaction sometimes triggered by the administration of contrast medium,6 is included among allergic complications, and major bleeding is defined by ISTH criteria10 and includes a drop of hemoglobin values of 20 g/L or more and/or intracranial bleeding. All of these conditions can occur within a few hours or up to several days after STEMI and are burdened with high mortality and morbidity when not recognized and adequately treated.2, 4-6, 11-13
By contrast, noncardiologic conditions are less likely and include SS and AC-induced TTS. These ones, similarly to the previous ones, may occur a few hours and up to several days after STEMI and are mainly due to oxygen supply and demand imbalance and to an absolute or relative cortisol deficiency associated with a toxic effect of catecholamines on the myocardium, respectively.8, 12, 14
The main features and differences of each condition described above are summarized in Table 2, while TTS, AC and AC-induced TTS after STEMI are discussed in more detail in the text below.
| Favorable factors | Unfavorable factors | ||
|---|---|---|---|
| Cardiac condition | |||
| Mechanical complications | Myocardial free wall rupture | Acute STEMI, chest pain, arterial hypotension and STE on ECG | Absence of muffled heart sounds, jugular venous distension and pericardial effusion on TTE |
| Procedural complications | Iatrogenic aortic dissection | Recent PCI, chest pain, arterial hypotension and STE on ECG | Absence of aortic regurgitation, pericardial effusion and intimal flap on TTE and CT scans |
| Acute stent thrombosis | Recent PCI, chest pain, arterial hypotension, STE on ECG and LVEF reduction associated with new RWMA on TTE | Absence of both fulfillment of type 1 myocardial infarction criteria and stent thrombosis while performing again the CA | |
| Hypersensitivity coronary artery disorder | Kounis Syndrome | Contrast medium injection, chest pain, arterial hypotension, STE on ECG and new RWMA on TTE | Absence of allergies and allergic stigmata. Normal flow over all coronary arteries while performing again the CA |
| Major bleedinga | Symptomatc bleeding with drop of hemoglobin values ≥ 20 g/L | Treatment with antiplatelet and anticoagulation agents, femoral artery access, arterial hypotension, MI and mental confusion. Sometimes STE on ECG | Absence of femoral access-site complications, drop of hemoglobin values ≥20 g/L and active source of bleeding on CT scans |
| Intracranial bleeding | Treatment with antiplatelet and anticoagulation agents, mental confusion, MI, STE on ECG and onset of new RWMA on TTE | Arterial hypotension, absence of neurological deficit and bleeding on CT scans | |
| Noncardiac conditions | |||
| Infection-related | Septic shock | Percutaneous access, arterial hypotension with low systemic vascular resistances, prerenal failure, MI, mental confusion. Sometimes STE on ECG | Arterial hypotension refractory to high-dose vasopressors. No elevation of inflammatory biomarkers, isolation of viruses or bacteria and sources of infection on laboratory exams, clinical evaluation and CT scans |
| Endocrinology disease-related | Adrenal crisis with adrenal crisis-induced Focal Takotsubo Syndrome |
Adrenal Crisis. Acute clinical deterioration, arterial hypotension associated with vasopressor-refractory shock that resolved over 2 h after hydrocortisone administration, recent discontinuation of prednisone, acute STEMI, low systemic vascular resistances, prerenal failure, hyponatremia, mental confusion, low values of serum cortisol. Adrenal Crisis with Adrenal Crisis-induced Focal Takotsubo Syndrome. Diagnosis of adrenal crisis with exclusion of other causes of shock, postmenopausal woman, STE on ECG, MI, LVEF reduction associated with new RWMA on TTE, partial recovery of both LVEF and RWMA after hydrocortisone administration, absence of complications during recovery |
N.A. |
- Abbreviations: CA, coronary angiography; CT, computed tomography; ECG, electrocardiogram; LVEF, left ventricle ejection fraction; MI, myocardial injury; N.A., not applicable; PCI, percutaneous coronary intervention; RWMA, regional wall motion abnormality; STE, ST-segment elevation; STEMI, ST-segment elevation myocardial infarction; TTE, transthoracic echocardiogram.
- a Defined by ISTH criteria; reference number.10
Takotsubo syndrome (or stress cardiomyopathy) is a STEMI-mimicking heart disease characterized by ECG changes, increased cardiac biomarkers, transient RWMA (showed by TTE or ventriculography) and sometimes shock.12, 15 Stress cardiomyopathy mainly affects postmenopausal women and is due to excessive sympathetic stimulation and/or catecholamine surges induced by emotional or physical factors, including endocrinological conditions, such as AC.7, 12, 15 Because RWMA are often STE-associated and STE (or ECG changes) are seen on multiple leads (beyond a single coronary artery distribution), to date, despite several diagnostic scores have been proposed, CA remains the gold standard diagnostic test to differentiate TTS from STEMI.15 Specifically, CA shows neither significant obstructive coronary artery disease nor acute plaque rupture over the RWMA-related coronary distribution areas. Based on the RWMAs, four main variants of TTS are recognized: apical (the most common and widely known), mid-ventricular, basal and focal.12, 15 The focal type has some peculiarities that differentiate it from the other forms: it shows a lower reduction of LVEF, RWMA and electrocardiographic changes are mainly localized to the midventricular septum and to anterolateral LV wall, and usually is characterized by a good prognosis.16 It should be noted that our patient showed all these features.
Adrenal crisis (or acute adrenal insufficiency) is a life-threatening condition arising from an absolute or relative deficiency of cortisol that requires prompt diagnosis and treatment. In adults, diagnosis of AC is clinical and requires the presence of an acute deterioration in health status associated with absolute or relative hypotension (systolic blood pressure < 100 mmHg or ≥ 20 mmHg lower than usual) that resolves within 1 to 2 h after parenteral glucocorticoid administration.17 Alongside these diagnostic features, the occurrence of one or more of the following is typical during AC: low systemic vascular resistances, mental confusion, electrolyte and/or biochemical abnormalities on lab exams, cortisol values lower than 10–15 mcg/dl and, sometimes, development of TTS7, 17. Treatment of AC requires fluids infusion and immediate administration of intravenous hydrocortisone, given as a 100 mg bolus, followed by 200 mg every 24 h, administered as a continuous infusion or as frequent intravenous (or intramuscular) boluses (50 mg) every 6 h, with subsequent doses tailored to the clinical response. If hydrocortisone is unavailable, another parenteral glucocorticoid may be used.17 AC may be triggered by several medical and/or pathophysiological conditions, including myocardial infarction.7, 8, 17 In this particular case, diagnosis of acute adrenal insufficiency is a real challenge, as it shares some clinical, hemodynamic, and biochemical features commonly found when a cardiogenic shock occurs, which must therefore be ruled out.
In our case, low circulating cortisol levels would underlie the development of AC with AC-induced fTTS. With regard to the development of AC, the recent short course of prednisone would have given a negative feedback on the hypothalamus-pituitary–adrenal axis resulting in insufficient cortisol production, while the acute STEMI would have led to the development of pro-inflammatory cytokines that, in turn, led to vasodilation and hypotension due to insufficient levels of circulating cortisol.17 Indeed, adequate levels of circulating cortisol are required to maintain normal vascular tone, as this hormone exerts both a synergistic action with catecholamines and a suppressive action on proinflammatory cytokines.17
The development of AC-induced fTTS, similarly to what has been reported by other Authors in the development of the other forms,8 could be explained by the insufficient circulating cortisol values as well. Specifically, both the synergistic action of cortisol with the myocardium in maintaining contractility and cardiac inotropism and its protective effect from catecholamines would be lacking, resulting in a reduction of regional kinesis.
Finally, although the development of AC-induced TTS is well known, to the best of our knowledge, this is the first case describing the development of the focal type of AC-induced TTS.
4 CONCLUSION
Cardiogenic shock is the most frequent complication after STEMI, however other rarer causes of shock may sometimes occur, including AC. The latter, in turn, may lead to the development of TTS. Although, to the best of our knowledge, cases of fTTS have never been reported yet, we describe a case of STEMI complicated by AC and, for the first time, AC-induced focal TTS.
AUTHOR CONTRIBUTIONS
Andrea Falcetta: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review and editing. Eleonora Bonfanti: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review and editing. Roberta Rossini: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review and editing. Giuseppe Lauria: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review and editing.
ACKNOWLEDGMENT
All authors contributed equally to the drafting, data collection, writing, revision, and approval of the manuscript.
FUNDING INFORMATION
All authors have nothing to disclose and had no sponsors or funding for the writing of the manuscript.
INFORMED CONSENT
Written informed consent had been obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data and original images are in the manuscript itself and are available from the corresponding author upon reasonable request.