Rupture of a previously undiagnosed intracranial aneurysm during endoscopic dacryocystorhinostomy: A case report
Abstract
Endoscopic endonasal dacryocystorhinostomy (EE-DCR) is an effective treatment for dacryocystitis. Aneurysmal rupture is generally not considered a complication of EE-DCR under general anesthesia. Here, we present a patient with intracerebral and subarachnoid hemorrhage secondary to the rupture of an undiagnosed intracranial aneurysm during EE-DCR. Clinicians should be aware of such fatal complications when using any vasoconstrictor intraoperatively.
1 INTRODUCTION
Endoscopic endonasal dacryocystorhinostomy (EE-DCR) is a safe and effective surgical approach for patients who suffer from chronic dacryocystitis.1 Normally, it is a relatively simple and short procedure. However, a rare and overwhelming complication of intraoperative intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) secondary to rupture of an undiagnosed intracranial aneurysm caused by hypertension due to submucosal injection of adrenaline can lead to life-long disability if not properly managed. Although intraoperative rupture of an unnoticed intracerebral aneurysm remote from a surgical site has been reported under other different scenarios,2, 3 to our knowledge, this event has not been reported previously.
2 CASE PRESENTATION
A 65-year-old female patient with persistent epiphora for 30 years was scheduled to undergo planned EE-DCR because of chronic dacryocystitis. She received a silicone tube intubation for nasolacrimal duct obstruction 8 years ago, and the symptom of epiphora was alleviated since then. However, the therapeutic effect did not last for a long time, and the symptom of epiphora aggravated 5 months ago.
Her past medical history was unremarkable except for irregular medication to control her long-term course of hypertension. Furthermore, she was not taking anticoagulants or antiplatelet agents. She had no other past medical issues or known congenital conditions. Her cardiopulmonary function was good, and her vital signs were stable. The baseline heart rate (HR) was 88 beats per minute (bpm), respiratory rate was 19 breaths/min, and non-invasive artery blood pressure (NIBP) was 159/87 mmHg.
Elective EE-DCR was performed under general anesthesia. After the patient arrived at the operating room, NIBP, continuous electrocardiography, and pulse oxygen saturation (SpO2) were monitored throughout the whole surgery. The initial NIBP was 143/87 mmHg, HR was 89 bpm, and SpO2 was 98% under room air. The following anesthetics for induction were injected via peripheral venous access: sufentanil 25 μg, midazolam 2 mg, cisatracurium 12 mg, propofol 60 mg, and lidocaine 60 mg. Oral tracheal intubation was performed successfully with a reinforced tracheal tube after 5 min of denitrogenation by preoxygenation. Both anesthesia induction and oral tracheal intubation were successfully established. The systolic blood pressure fluctuated between 90 and 120 mmHg during these periods. Anesthesia was maintained via continuous infusion of remifentanil combined with sevoflurane. Approximately 2 min after the operation started, the NIBP suddenly increased to 244/130 mmHg in a few seconds after the submucosal injection of the suspension of tetracaine and adrenaline (1:20,000), accompanied by increased HR (151 bpm). The attending anesthesiologist called for stopping the operation immediately. Propofol (100 mg) was administered intravenously at the same time. The time interval of NIBP measurement was adjusted from 3 min to continuous measurement. To quickly address the clinical manifestation of sympathetic symptoms as soon as possible, several repeated boluses of esmolol 30 mg and nicardipine 0.5 mg to a total of 90 mg and 1.5 mg, respectively, were injected intravenously. Simultaneous invasive arterial blood pressure was monitored urgently after radial artery cannulation. After discussion with the surgeons, the surgery proceeded as hemodynamics gradually stabilized. The suspension of tetracaine and adrenaline was abandoned since then.
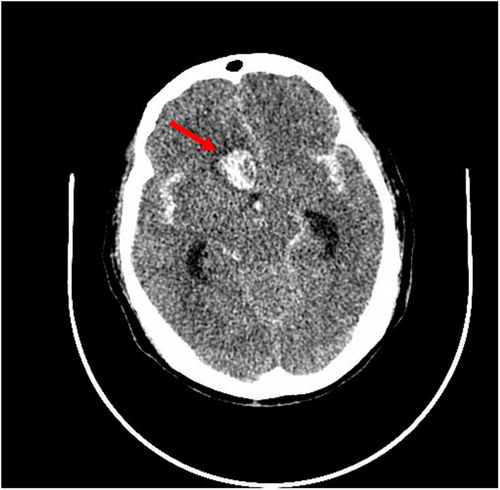
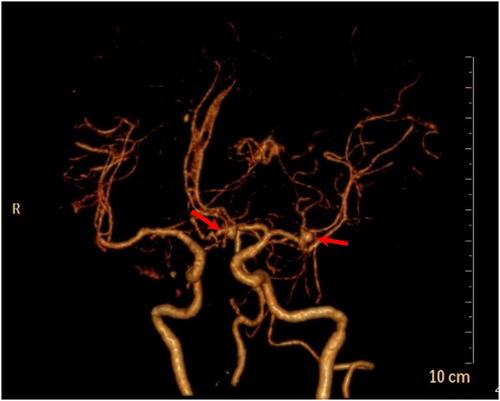
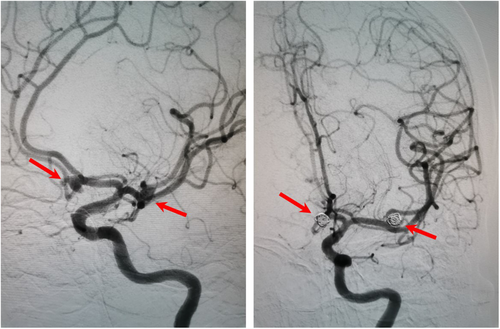
Approximately 40 min later, the patient was transferred to the postanesthesia care unit with tracheal intubation for recovery. Approximately 30 min later, the patient gradually woke up, but her consciousness was still in a superficial coma. Confusingly, she cannot lift her right leg. Considering the huge hemodynamics fluctuance intraoperatively, cerebral vascular accidents cannot be ruled out. To clarify the confusion, an urgent bedside head computed tomography (CT) scan was performed immediately. There was a patchy high-density shadow with a size of approximately 26 mm * 17 mm * 25 mm (CT value of 77 HU) in the right frontal lobe (Figure 1). A ring-shaped low-density shadow was around it. The adjacent brain parenchyma was compressed, while the midline structure was not shifted. In addition, high-density patchy shadows were seen in the bilateral ventricles and the third and fourth ventricles. Strip-like high-density shadows were also seen in the sellar region, bilateral fissure cistern, and cerebral sulcus. A diagnosis of ICH and SAH was established. It is suspected that a rupture of an unidentified preexisting aneurysm could be responsible for those findings. Subsequently, the patient received digital subtraction angiography, which demonstrated saccular aneurysms of the A1 segment of the left anterior cerebral artery and the M1 segment of the left middle cerebral artery (Figure 2). No other causes of SAH were found. Endovascular coil embolization of the aneurysms (Figure 3) combined with external ventricular drainage was then performed, and the patient was transferred to our neurologic intensive care unit after those interventions. She received systemic internal chemotherapy, sedation, and osmotherapy with mannitol. Bloody cerebrospinal fluid (CSF) was drained out accordingly under intracranial pressure (ICP) monitoring. After a week of treatment and rehabilitation, the condition of the patient gradually improved. She returned to her previous state of consciousness and muscle strength and was discharged 15 days after EE-DCR. Now, she has returned to her normal life as it was before the surgery.
3 DISCUSSION AND CONCLUSION
Endoscopic endonasal dacryocystorhinostomy can be considered a safe procedure, with a low overall rate of complications. Intraoperative complications of EE-DCR include hemorrhage, orbital wall injury, CSF leakage, and injury to the canaliculi.4 When properly performed by experienced surgeons, EE-DCR should not result in any significant intraoperative complications. It is also a low-risk surgical type for anesthesiologists. However, aneurysmal rupture is generally not considered a complication of EE-DCR under general anesthesia. Intra-aneurysmal pressure is equal to the systemic blood pressure. Transmural pressure can be defined as the difference between blood pressure and ICP.5 Therefore, the increased blood pressure and decreased ICP or the weakening of the aneurysmal wall could disrupt this balance. The decreased ICP, such as ventricular drainage, could induce SAH during endoscopic third ventriculostomy.6 Recently, we encountered a case of an undiagnosed underlying aneurysmal rupture, which is associated with hemodynamic changes and sympathetic hyperactivity. As a consequence, a sudden rapid increase in blood pressure caused a hypertensive ICH due to the rupture of a penetrating artery weakened by chronic artery hypertension. As we mentioned previously, the patient has a long-term course of chronic hypertension, which is poorly controlled.
According to a previous report, approximately 3.6–6% of the general population harbors an unruptured intracranial aneurysm.7 Although the incidence is low, the rupture of an underlying unknown aneurysm perioperatively must be considered a potential complication. In the present case, we did not perform any preoperative assessment of the cerebrovascular vessels. We underlooked this case because she had no other signs or symptoms of cerebrovascular disease, except her female gender and hypertension. Female gender and hypertension are both considered to be the two risk factors for aneurysm formation and rupture.7 This acquired anomaly can be associated with a long-term course of high blood flow dynamics and abnormal CSF due to irregular unstable hypertension. However, congenital cranial abnormalities in this patient could not be ruled out.
Avoiding such fatal complications is vitally important for otorhinolaryngologists and anesthesiologists. Several advices may help to decrease intraoperative major adverse cardio- and cerebrovascular events. First, to decrease intraoperative bleeding, mucosal decongestion by topical vasoconstrictors and/or injection of vasoconstrictors are generally used before starting the procedure. However, the concentration of adrenalin should be appropriate. In our case, a concentration of 1:20,000 adrenalin was injected at the level of the lateral nasal wall, which was ten times the standard dose (1:200,000). Dilutional errors very often end up as “near miss” events, but consequences may have been different due to the presence of aneurysms. Second, try to not submucosal injection of vasoconstrictor as much as possible. Instead, otorhinolaryngologists could place pledgets with 1:5000 adrenalin or oxymetazoline 0.05% on the mucosal surface.8 Third, if a submucosal injection is necessary, it must be pumped back before injection because the nasal mucosa has a copious blood supply. It is a pity that we have not obtained any solid and objective evidence, such as a drug concentration test of the blood sample. Fourth, vigilance should be maintained when using any vasoconstrictor intraoperatively. When sympathetic excitement begins to appear, the medication should be ceased immediately. Fifth, preoperative brain angiopathy is recommended to be aware of any undiagnosed underlying cerebrovascular malformation for high-risk patients.
If the decision is made to treat systemic hypertension, the choice of an antihypertensive agent is important. Vasodilating drugs, such as nitroprusside, nitroglycerin, and nifedipine, can be expected to increase ICP and may reflexively increase plasma catecholamines, which may be deleterious to the marginally perfused injured brain. Sympathomimetic-blocking antihypertensive drugs, such as β-blocking drugs (labetalol, esmolol) or central acting α-receptor agonists (clonidine), are preferred because they reduce blood pressure without affecting the ICP.9, 10 In our case, propofol, esmolol, and nicardipine were administered to antagonize sympathetic excitement caused by adrenaline. Those agents with a short half-life time have an advantage when the blood pressure is labile. Unfortunately, the intracranial aneurysm still ruptured even though hypertension lasted for a short period.
Similar to our case, Kyung et al11 reported a case of delayed spontaneous aneurysm rupture after ear surgery. Their case emphasized the need for awareness of severe delayed complications after ear surgery. We believe our case could serve as a compelling reminder that although ear–nose–throat surgeries are usually low-risk and short, clinicians must be aware of other potentially fatal complications. What we have experienced may help other anesthesiologists to improve their clinical practice when faced with such situations.
AUTHOR CONTRIBUTIONS
Zhang MQ involved in writing–original draft. Xin W involved in writing–review and editing. All authors have read and approved the manuscript.
ACKNOWLEDGMENT
Not applicable.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICAL APPROVAL
This study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Research
DATA AVAILABILITY STATEMENT
All data generated during this study can be accessed through direct communication with the corresponding author and the agreement of all research team members.