Negative effects of rivaroxaban observed in a patient with multiple common risks triggered by a bicycle accident
Abstract
The negative effects of anticoagulants are well-known in patients with renal impairment, drug–drug interaction, lower physical conditions, and multiple comorbidities. We highlight that even in patients with controllable multiple risks for rivaroxaban use, add-on inevitable risks will lead to negative effects greater than those expected.
1 INTRODUCTION
The negative effects of anticoagulants are well known in patients with renal impairment, drug–drug interaction, lower physical conditions such as elderly individuals, and multiple comorbidities.1, 2 In clinical settings, patients have not only common existing evitable risks but also inevitable multiple risks, maintaining the balance between risks and benefits.
Herein, we present a patient with several common risks associated with the use of rivaroxaban, a selective inhibitor of Factor Xa. An inevitable risk of bicycle accident triggered the negative effects of rivaroxaban, leading to a delay in the healing of wound, which became severely necrotic. We aim to highlight that even in patients with controllable multiple risks, add-on inevitable risks will lead to negative effects greater than those expected.
2 CLINICAL CASE
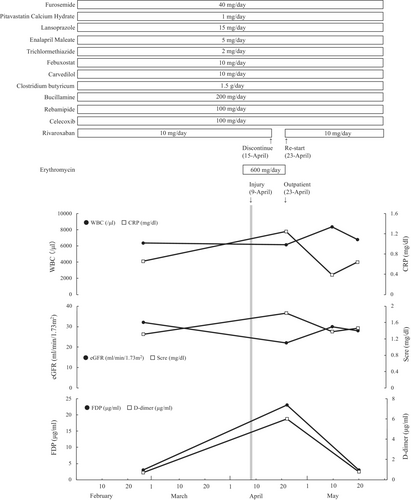
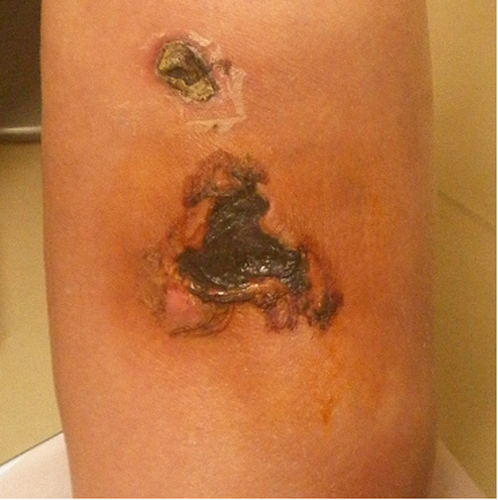
A 60-year-old woman (79 kg, 151 cm) with stage G3b chronic kidney disease, atrial fibrillation (AF), heart failure, bronchial asthma, and rheumatism visited the cardiology, rheumatology, and respiratory medical departments for several years at our university hospital. On April 6, 2020, she started sinusitis treatment with 600 mg/day of erythromycin, a potent cytochrome P450 (CYP) 3A4 inhibitor, prescribed by the respiratory department (Figure 1). Four days after starting erythromycin administration, she fell after riding a bicycle due to loss of balance and injured her left lower leg. She visited an orthopedic clinic near her home and observed a delay in wound healing due to epidermolysis. On day 6 after the injury, the orthopaedist discontinued rivaroxaban and recommended her to visit our hospital for anticoagulation examination and careful health monitoring. On day 14 after the injury, the patient visited the dermatologist and was diagnosed with black necrotic tissue, based on the results of computerized tomography. She presented a 10 cm subcutaneous hematoma and 2- and 4 cm cutaneous ulcers (Figure 2). On the same day, rivaroxaban treatment was re-started because of the following. (1) The dermatologist's diagnosis indicated no active bleeding, and the subcutaneous hematoma had not reached the muscle layer; moreover, hematoma expansion was not observed. (2) Pharmacists recommended the discontinuation of erythromycin to the pulmonologist. Her laboratory results, except those of the coagulation test and inflammatory response, did not change throughout the period (Figure 1). In the period, she received treatment for the injury in the outpatient department. Finally, her injury healed under a plastic surgeon changed of type of dressings and did debridement, and her medication was continued.
3 DISCUSSION
Our patient with multiple common risks for the negative effects of rivaroxaban experienced an inevitable risk of the bicycle accident, which led to a delay in the healing of the injury and became severely necrotic. It is necessary to consider risk–benefit balance for bleeding risk thrust by rivaroxaban use and thrombotic risk during discontinuing rivaroxaban. Our re-start timing of rivaroxaban may suitable according to the dermatologist's diagnosis of our case. We emphasize that there are multiple common risks associated with rivaroxaban use in this case.1, 2
Several previous studies, including from our group, have reported that rivaroxaban interacts with other drugs. She was co-administered rivaroxaban with celecoxib. Briefly, the combination of celecoxib, a nonsteroidal anti-inflammatory drug, and rivaroxaban has been reported to cause bleeding according to a spontaneous reporting system for adverse events in Japan.3 On the contrary, bleeding was not observed during celecoxib and rivaroxaban coadministration in 3290 AF patients.3 Therefore, we think the combination therapy may not or does not increase bleeding risk and delay in wound healing in our case. Moreover, she was co-administered erythromycin with rivaroxaban. Erythromycin has been shown to increase the blood concentration of rivaroxaban by 1.3-fold via CYP3A4 inhibition.1 Therefore, this drug–drug interaction may have had a impact on injury healing in this case.
She had been administered 10 mg of rivaroxaban daily over several years because her estimated glomerular filtration rate (eGFR) was over 30 ml/min/1.73m2. An eGFR under 30 ml/min/1.73m2 was only observed in the discontinuing period of rivaroxaban. As per injury healing and thrombotic risk, we re-started rivaroxaban for 10 mg daily. After re-starting rivaroxaban, eGFR did not worsen and was maintained over 30 ml/min/1.73m2.
Rivaroxaban has been associated with complications of bleeding and infections after surgery, and thus, delay in wound healing.4 In our patient, the loss of balance, which led to the bicycle accident, was an inevitable trigger for the negative effects of rivaroxaban. We assessed the phenomenon with the following time course: (1) the wound got worse when rivaroxaban was administered and (2) the wound healed after stopping rivaroxaban. Therefore, we assessed that rivaroxaban use may be the main reason for the delay in wound healing.
On the contrary, necrosis in our patient may have been caused by insufficient blood flow after the accident by potentially existing arteriosclerosis because she has multiple risks for arteriosclerosis such as chronic kidney disease, hypertension, and rheumatoid arthritis over 10 years.5, 6 This suggests that rivaroxaban triggered a delay in wound healing by bleeding, and the wound became necrotic due to arteriosclerosis. Even if the patient had not received erythromycin, the use of which is an evitable risk factor, the patient might have taken more time to heal considering her age, multiple comorbid conditions, and baseline health condition.
4 CONCLUSION
We reported a case in which a patient experienced the loss of balance while riding a bicycle, which resulted in injury. Due to the use of rivaroxaban, underlying drug–drug interactions, and multiple risk factors that may lead to bleeding or impaired healing, she experienced negative outcomes from her injury. In patients with controllable multiple risks for rivaroxaban use, add-on inevitable risks will lead to negative effects greater than those expected.
AUTHOR CONTRIBUTIONS
Kurumi Abe: Conceptualization; investigation; writing – original draft; writing – review and editing. Kenji Momo: Project administration; writing – original draft; writing – review and editing. Yuji Oto: Visualization; writing – review and editing. Masaki Kida: Investigation; resources; writing – review and editing. Takuma Iwakiri: Investigation; resources; writing – review and editing. Katsumi Tanaka: Supervision; writing – review and editing. Tadanori Sasaki: Supervision; writing – review and editing.
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
TS received an honorarium for presentation at Daiichi Sankyo, NIPRO, Nichi-Iko, Pfizer, Sandoz, Mylan, and Meiji Seika Pharma. As a potential conflict of interest, KM received honorarium fees for presentations from Nippon-Kayaku and Abbvie. The Department of Hospital Pharmaceutics, School of Pharmacy, Showa University received budget from Ono with a contract research project according to the collaborative research agreement. In addition, Hospital Pharmaceutics received a research grant from Daiichi Sankyo, Mochida, Shionogi, Ono, Taiho, and Nippon-kayaku. The other authors declare no conflict of interest associated with this manuscript.
ETHICAL APPROVAL
None.
CONSENT
We obtained written informed consent for publication from the patient.
Open Research
DATA AVAILABILITY STATEMENT
All data is contained in the manuscript.