Diagnosis and treatment of flurbiprofen-induced Stevens–Johnson syndrome: A rare case report
Abstract
Our case highlights the occurrence of severe cutaneous adverse reactions with flurbiprofen use and alerts physicians to its odds with safer drugs.
1 INTRODUCTION
Flurbiprofen, a promptly accessible nonsteroidal anti-inflammatory drug (NSAID), is broadly used as an effective antipyretic and analgesic. Like other NSAIDs, it inhibits the cyclooxygenase enzymes, thus blocking the synthesis of prostaglandins, thromboxane, and prostacyclin. Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are uncommon but severe mucocutaneous hypersensitivity reactions and are most commonly linked with medication use.1-3 These start with influenza-like prodrome and rapidly progress to severe mucocutaneous lesions and skin sloughing, culminating in a medical emergency that can be fatal.4 Here, we report an infrequent incidence of flurbiprofen-induced SJS in a 30-year-old Asian female.
2 CASE PRESENTATION
A 30-year-old female patient presented to the emergency with complaints of widespread red macules and hematemesis for 2 days. Her illness started with a prodrome of throat pain and high-grade continuous fever (102°F) followed by itching and dryness of both eyes that worsened gradually. Initially, she had a burning sensation on her lips. By the 14th day, her face, lips, and eyes started to swell to the extent that she could not open them. On the 15th day, multiple red lesions appeared initially on the face and neck with rapid spread to the trunk and arms but sparing the scalp, entire lower limbs, and palms. Also, she had four episodes of hematemesis on the same day. Her drug history revealed that she has been taking Tab Fluribiprofen 100 mg TDS for the last 16 days, and she reported the symptoms on the 14th day of Flurbiprofen use. Her past and family history were insignificant for any allergic reactions.
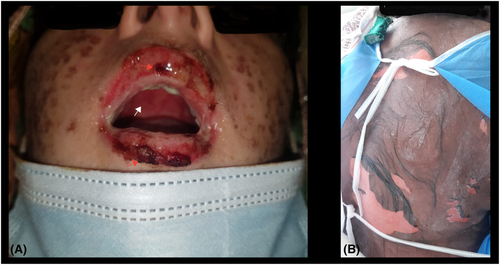
Her blood pressure was 130/80 mmHg, pulse was 105 beats per minute, temperature was 101°F, and SpO2 was 97%. Widespread confluent erythematous macules were noted that were painful and tender, consistent with atypical flat targets. Her lips had dark, crusted lesions, and the oral mucosa was red and inflamed (Figure 1A). Later, epidermal detachment occurred, which was most prominent on the back (Figure 1B). Ocular examination showed conjunctival congestion and membrane formation on the tarsal conjunctiva of both eyes.
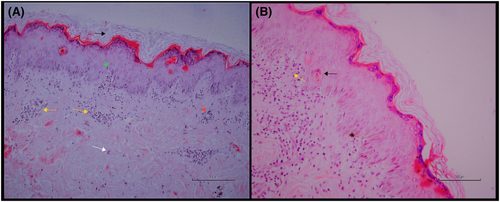
Her laboratory investigations (Table 1) revealed elevated ESR, CRP, and total bilirubin. Skin biopsy showed necrotic changes in the keratinocytes and lymphocytic infiltrate in the dermis (Figure 2). Based on the clinical presentation and skin biopsy findings, we diagnosed the patient with flurbiprofen-induced SJS.
| Investigations | Results | Reference range |
|---|---|---|
| TLC (cells/mm3) | 7800 | 4000–11.000 |
| DLC | N 81 L 14.5 | |
| Hb (g/dL) | 12.9 | 11.5–17.5 |
| Platelets (cells/mm3) | 109.000 | 150.000–450.000 |
| Creatinine (mg/dL) | 0.8 | 0.6–1.2 |
| Serum Urea (mg/dL) | 31.4 | 18–45 |
| Serum Sodium (mmol/L) | 138.7 | 135–150 |
| Serum Potassium (mmol/L) | 3.08 | 3.5–5.1 |
| Serum LDH (U/L) | 243 | 140–280 |
| Serum Ferritin (ng/mL) | 114.5 | 30–400 |
| ESR (mm/Ist hour) | 54 | 1–13 |
| CRP (mg/L) | 63.85 | 0–5 |
| Total bilirubin (mg/dL) | 1.6 | 0.1–1.0 |
| ALT (U/L) | 35.1 | 10–50 |
| ALP (U/L) | 50 | 40–129 |
| PT (seconds) | 17 | 11–13.5 |
| APTT (seconds) | 34 | 21–35 |
| HIV, HBsAg, HCV | Negative | |
| Culture of Blister's Aspirate | No growth |
- Abbreviations: ALT, alanine transaminase; APTT, activated partial thromboplastin time; AST, Aspartate transaminase; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; Hb, hemoglobin; LDH, lactate dehydrogenase; PT, prothrombin time; TLC, total leucocyte count.
The patient was managed in the intensive care unit for 8 days, where all previous medicines were stopped. She received intravenous fluids, nasogastric tube feeding, injection Ceftriaxone 2 gm IV once a day for 6 days, injection Pheniramine 25 mg IV twice a day for 4 days, injection Dexamethasone IV 0.1 mg/kg body weight for 6 days, infusion Paracetamol IV, topical eye lubricants and Fluorometholone plus neomycin eye drops, liquid paraffin for wound care. Also, the conjunctival pseudomembranes were peeled off under local anesthesia. The patient showed significant improvement as the skin lesions gradually healed, the swelling decreased and body temperature normalized. She was educated regarding drug use to prevent recurrence and was discharged with a follow-up appointment.
3 DISCUSSION
SJS, SJS-TEN overlap, and TEN are uncommon; however, hazardous severe cutaneous adverse reactions (SCARs) with an incidence of 0.05–2 cases per million population per annum.1, 2 The RegiSCAR study reported an overall mortality rate of 23% at 6 weeks and 34% at 1 year.5
Drugs are the most common triggers accounting for more than 65% of the cases.3 The high-risk drugs include anti-epileptics (phenobarbital, phenytoin, carbamazepine, and lamotrigine), sulfonamides, NSAIDs, nevirapine, and allopurinol.6 HIV, HSV, Mycoplasma pneumoniae, and Lupus erythematosus are less common but notable triggers.3, 7-10 The possible risk factors that make the individual susceptible to drug-induced reactions include certain HLA haplotypes, large doses of medicines, herpes simplex virus, Ebstein–Barr virus, and varicella zoster virus.4, 11 The underlying pathogenesis is not clearly understood, but the process is T-cell-mediated.4
Oxicam derivatives carry the highest risk among the NSAIDs, while other NSAIDs have a significantly lower association.12 Though propionic acid derivatives (flurbiprofen, ibuprofen) have a very low risk, they can be the potential culprits. For instance, a retrospective study conducted at university hospitals in Korea between 2010 and 2015 recorded 170 cases of NSAIDs induced SCARs (85 of SJS, 32 of SJS/TEN overlap, and 17 of TEN) where propionic acids were the major culprits (68 cases total).13 A single case with ibuprofen use, in particular, has been reported from Nepal.14 Similarly, our case is one of the few incidences described so far and one more addition to the literature.
The clinical manifestations usually start within 4 days–4 weeks of drug use with a prodrome of fever, malaise, myalgia, photophobia, and itching and burning of the eyes.4, 11 After 1–3 days, erythematous flat macules with dark centers that are painful and tender appear initially on the face and trunk and then spread to other areas, typically sparing the scalp, palms, and soles.11 Later, the lesions transform into vesicles and bullae with epidermal sloughing. Mucosal involvement may precede or follow the skin eruptions and is seen in more than 90% of cases.11 Usually, two or more mucosal membranes are involved, with oral mucosa (stomatitis and dysphagia) being the most common, followed by ocular mucosa (pain, conjunctivitis, purulent discharge), and urogenital mucosa (urethritis, genital erosions).4, 11 Our patient had a similar presentation with widespread mucocutaneous involvement 1 day after typical prodromal symptoms. Re-epithelialization begins several days after the acute stage (lasts 8–12 days) is over and requires 2–4 weeks.4, 11
Both SJS and TEN result from a similar underlying process and are differentiated based on the degree of skin involvement; SJS involves less than 10% of the total body surface area (BSA), SJS/TEN overlap involves 10%–30% of the BSA, and TEN involves more than 30% of the BSA.11 There is no specific diagnostic tool, and the diagnosis mainly rests on clinical presentation supported by the biopsy. Our diagnosis was confirmed on the same parameters with a typical clinical picture, normal routine investigations, and supportive histologic findings.
The management mainly focuses on supportive measures, including fluid and electrolyte balance, tube feeding, temperature regulation, pain relief, infection prevention, and sterile handling and disinfection of the affected skin.4 Medical therapies, including systemic glucocorticoids, cyclosporine, tacrolimus, and IV immunoglobulin, have been used in small cohorts, but their role is variable and unclear.15 The case under consideration received glucocorticoids in addition to supportive therapies.
Long-term complications may involve the skin (hypo or hyperpigmentation, scarring, eruptive nevi), eyes (pain, scarring, dryness, keratitis, and rarely blindness), mouth (xerostomia, caries), respiratory system (chronic bronchitis, bronchiectasis), and urogenital system (labial and vaginal adhesions, recurrent cystitis, urinary retention).4, 11
4 CONCLUSION
While prescribing drugs, even those generally deemed safer, the physicians should stratify the risk based on underlying risk factors, HLA haplotypes, doses, and multiple drug regimens. Due to their genetic predispositions and peculiar drug utility patterns, certain individuals can be the potential victims of flurbiprofen-induced severe cutaneous adverse reactions (SCARs). Moreover, the low risk with a drug should not underestimate the clinical picture and delay the diagnosis and management.
AUTHOR CONTRIBUTIONS
AAK, FR, FUK, TT, and SA prepared the original draft and reviewed and edited the manuscript's final version. AA critically reviewed and edited the manuscript's final version.
ACKNOWLEDGMENTS
We would like to thank Dr. Mehran Khan, Assistant Professor of Dermatology, Khyber Teaching hospital Peshawar and Dr. Bushra Nasib, Lecturer Pathology department Khyber Medical University Peshawar, for helping with obtaining and analyzing the Biopsy specimen.
FUNDING INFORMATION
The authors did not receive any funding for this work.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
GUARANTOR
AAK is the Guarantor.
Open Research
DATA AVAILABILITY STATEMENT
All the data has been provided in the manuscript.