Pseudoxanthomatous salpingitis: An uncommon lesion of the fallopian tube
Abstract
Pseudoxanthomatous salpingitis (PXS) is an uncommon condition characterized by the presence of pigment-laden histiocytes within the lamina propria of the fallopian tube. Less than 30 cases of PXS have been reported in the literature. We herein report a case of PXS associated with an endometriotic cyst.
1 CLINICAL IMAGE
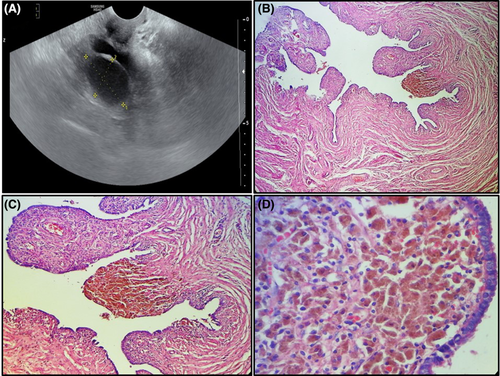
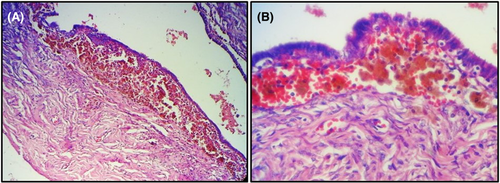
A 49-year-old woman gravida 4 para 4, presented with a two-month history of pelvic pain. Physical examination did not disclose pelvic tenderness or palpable masses. Pelvic ultrasound examination showed fluid-filled dilatation of the left fallopian tube and a bilocular heterogeneous cyst of the left ovary measuring 4.3 × 4.9 cm (Figure 1A). The patient underwent left salpingo-oophorectomy. Grossly, the ovarian cyst was filled with brown material and the fallopian tube was dilated. Histologically, we noted the presence in the lamina propria of the fallopian tube of numerous pigmented histiocytes (Figure 1B–D). Their cytoplasm was filled with coarse cytoplasmic granules of hemosiderin appearance.1 There were a few foamy histiocytes with vacuolated cytoplasm. Some lymphocytes were intermingled with the histiocytes. The ovarian cyst was lined by an endometrial epithelium with underlying endometrial stroma.1 The latter contained aggregates of pigmented or hemosiderin-laden histiocytes (Figure 2A, B). The final pathological diagnosis was PXS associated with an endometriotic cyst. The postoperative course was uneventful. Currently, the patient is monitored on an outpatient basis with a follow-up period of 2 months.
AUTHOR CONTRIBUTIONS
Dr Faten LIMAIEM and Dr Ahmed HALOUANI prepared, organized, wrote, and edited all aspects of the manuscript. Dr Faten LIMAIEM prepared all of the histology figures in the manuscript. Pr Kaouther DIMASSI participated in the conception and design of the study, the acquisition of data, analysis, and interpretation of the data. All authors contributed equally to preparing the manuscript and participated in the final approval of the manuscript before its submission.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST
None declared.
ETHICAL APPROVAL
All procedures performed were in accordance with the ethical standards. The examination was made in accordance with the approved principles.
CONSENT
Published with written consent of the patient.
Open Research
DATA AVAILABILITY STATEMENT
In accordance with the DFG Guidelines on the Handling of Research Data, we will make all data available upon request.