Acetaminophen-induced Stevens–Johnson syndrome with lethal lung injury: A case report
Abstract
Stevens–Johnson syndrome (SJS) with respiratory distress can lead to fatal outcomes. However, there are a few reports of drug-induced lung injury with diffuse alveolar damage caused by acetaminophen, the most severe type. Here, we describe a fatal case of acetaminophen-induced SJS in a child with irreversible lung lesions.
1 INTRODUCTION
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe systemic diseases with cutaneous and mucosal lesions and are characterized by a polymorphous vesicular and bullous eruption with fever triggered by drug administration or infection.1 SJS causes complications in many organs, such as the liver, kidneys, and lungs, leading to treatment difficulties.1, 2 In particular, patients with respiratory distress can require extended treatment or have fatal outcomes.2 However, there are few reports of SJS with lung lesions in children.
Acetaminophen is an antipyretic commonly used to relieve fever and associated discomfort in children. A prospective cohort study suggested that as many as 95% of children were exposed to acetaminophen by the age of 9 months.3 Although not adequately recognized, acetaminophen is a drug that can easily cause SJS. Acetaminophen was reported as one of the top five most frequently administered drugs associated with SJS/TEN in Japan and the United States.4, 5 According to a recent review of cases of SJS/TEN and SJS/TEN overlap suspected to be associated with acetaminophen, 77.8% received supportive and symptomatic care. Systemic corticosteroids and intravenous immunoglobulin were administered to 69.4% and 44.4% of patients, respectively. The prognosis of these patients was not poor, as all patients survived, although long-term sequelae were available in only 13.9%.6
Herein, we describe a fatal case of acetaminophen-induced SJS in a child with irreversible lung lesions.
2 CASE REPORT
A 6-year-old boy with fever was suspected of having Mycoplasma pneumoniae infection and was prescribed acetaminophen and clarithromycin in the clinic 7 days before admission. He had no previous medical or family history. In addition, there was no history of drug allergy or exposure to suspected drugs before disease onset. At night, erythematous rashes appeared on the patient's face and trunk. Three days before admission, he was diagnosed with SJS at a general hospital based on skin findings. Although he was administered prednisolone (2.0 mg/kg/day), his erythema gradually expanded and developed dyspnea. The patient was transferred to our hospital for further intensive care.
Upon admission, his vital signs were as follows: body temperature, 37.3°C; blood pressure, 86/52 mmHg; heart rate, 86 beats/min; respiratory rate, 36 breaths/min; and oxygen saturation, 90% with O2 at 8 L/min via an oxygen mask with a reservoir bag. Retractions appeared immediately below the neck and under the breast. Subcutaneous emphysema was observed in the cervical and supraclavicular fossae. Chest examination revealed coarse crackles, decreased breathing sounds, and bilateral expiratory wheezing. No hyperemia or erosion was found in the conjunctiva of the eyeball, bloody scabs were found on the lips, and no redness or erosion was found in the oral cavity. The body surface area of blisters or erosions was <10%, and that of erythema was 25% (Figure 1A). Laboratory examination further revealed the following findings: white blood cell, 5800/μl (neutrophils 69.6%, lymphocytes 19.3%, and eosinophils 0%); red blood cells, 482 × 104 /μl; hemoglobin 13.2 g/dl; platelet, 35.2 × 104/μl; total-bilirubin, 7.0 mg/dl; aspartate aminotransferase, 304 U/L; alanine aminotransferase, 372 U/L; lactate dehydrogenase, 614 U/L; ferritin, 615 ng/ml; soluble interleukin-2 receptor, 779 U/ml; Krebs von den Lungen 6 (KL-6), 953 U/ml; and antinuclear antibody titer, 1:40. The drug lymphocyte stimulation test (DLST) was positive for acetaminophen (H-thymidine, 905 cpm; stimulation index, 274%) and negative for clarithromycin (H-thymidine, 226 cpm; stimulation index, 114%). His HLA analysis showed HLA-A *02:07, *24:02, HLA-B *46:01, *52:01 alleles. Moreover, the loop-mediated isothermal amplification (LAMP) assay for M. pneumoniae was negative, and there was no increase in antibody titer. Chest radiography revealed subcutaneous emphysema, bilateral mediastinal emphysema, and decreased permeability in the bilateral middle and lower lung fields (Figure 1B).
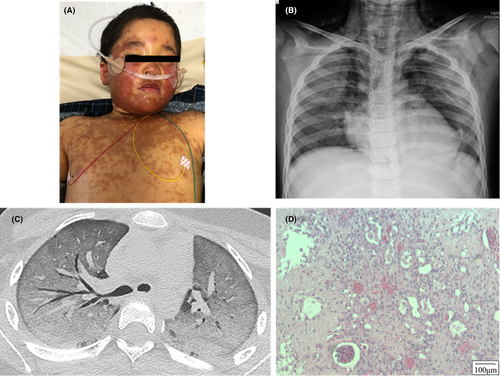
Based on these findings, we diagnosed severe SJS due to acetaminophen with mediastinal/subcutaneous emphysema. We also administered high-dose immunoglobulin (0.5 g/kg/day, for 5 days) and methylprednisolone pulse therapies (1 g/day for 3 days/week), resulting in an improvement in the eruption. However, his respiratory failure persisted, and the chest CT showed a ground-glass-like shadow (Figure 1C). We diagnosed acute respiratory distress syndrome (ARDS), and cyclosporine (3.0 mg/kg/day) was added to the treatment regimen on Day 9. Artificial respiration with tracheal intubation was initiated on Day 13. Persistent progressive respiratory failure, veno-venous extracorporeal membrane oxygenation, and plasmapheresis were introduced on Day 44. On Day 51, left hemopneumothorax developed, and thoracoscopic hemostasis and lung biopsy were performed. Pathological examination of the pulmonary tissue revealed the diffuse alveolar injury, confirming the diagnosis of diffuse alveolar damage (DAD; Figure 1D). Although we performed various multidisciplinary treatments, his lung lesions showed poor treatment responsiveness, and he died on Day 90.
3 DISCUSSION
Stevens–Johnson syndrome/TEN is caused by a type IV-c delayed hypersensitivity reaction to infections or drugs.7 SJS/TEN is defined as a widespread vesiculobullous rash with epidermal sloughing, necrosis, and mucous membrane involvement, typically in the eyes, oral cavity, and skin. SJS/TEN were classified according to the extent of the detached and detachable epidermis, expressed as a percentage of the total body surface area (TBSA) (SJS: <10% TBSA; SJS-TEN overlap: 10%–30% TBSA; and TEN: >30% TBSA).8 Mucosal and eye inflammation are present in 90% and 60% of cases, respectively. Severe cases culminate in corneal scarring, respiratory distress syndrome, pneumonia, and respiratory failure.9
According to the United States Food and Drug Administration Adverse Event Reporting System database, the top five most frequently administered drugs associated with SJS/TEN are valdecoxib, lamotrigine, phenytoin, acetaminophen, and furosemide.5 In contrast, allopurinol, loxoprofen, acetaminophen, carbamazepine, and lamotrigine are the frequent causes of SJS in Japan.4
In our case, we initially suspected that it was caused by an infectious agent, such as mycoplasma, or was induced by drugs such as acetaminophen and clarithromycin. However, the LAMP assay of M. pneumoniae was negative, and the DLST against acetaminophen was positive; thus, acetaminophen was considered to be the cause.
Stevens–Johnson syndrome caused by acetaminophen tended to be more predominant in the younger groups aged 0–19 years old, with a median time to onset of 2 days.4 The time to onset was only 1 day in the present case, which is consistent with a previous report. However, the present case was followed by a more rapid and serious course than that of the previous reports on acetaminophen-related SJS. Therefore, early and aggressive therapeutic interventions are required, as in this case.
A recent review of cases of SJS/TEN associated with acetaminophen summarized a total of 36 patients described in 29 previously published reports.6 TEN, SJS, and SJS/TEN overlap were diagnosed in 24 (66.7%), 10 (27.8%), and 2 (5.6%) patients, respectively. Of these 36 patients, 28 (77.8%) received supportive and symptomatic care. Systemic corticosteroids were administered to 25 patients (69.4%), and intravenous immunoglobulin was administered to 16 patients (44.4%). The prognosis of these patients was not poor, as all patients survived according to the reports, although long-term sequelae were available only in five patients (13.9%).6
Reports in the United States have shown that the SJS mortality rate was 4.8% in adults10 and 0% in children.11 Predictors of mortality risk in pediatric SJS include the presence of renal failure, sepsis, epilepsy, bacterial infections, and malignant diseases.11 According to this criterion, the mortality risk was not high in our case. Although the patient's skin and mucosal lesions improved with immunosuppressive therapy, the lung injury did not improve despite intensive treatment. Accordingly, we considered that the severity of lung injury might also affect the prognosis of SJS, with a profound impact on mortality. Additional clinical studies with larger numbers of patients are needed to determine whether a pulmonary impairment is a risk factor.
In adults, 37.8% of patients with SJS/TEN may require mechanical ventilation in the acute phase.12 Pathologically, epidermal sloughing of the bronchial epithelium was observed by fiberoptic bronchoscopy in 27% of SJS/TEN cases, and those with positive pathological findings showed early pulmonary complications in the acute phase, with a high rate of fatal outcome.13 Although fiberoptic bronchoscopy could not be performed in our case, pathological findings of pulmonary tissue in the lung biopsy revealed DAD. There are few reports of drug-induced lung injury caused by acetaminophen and even fewer reports of DAD caused by acetaminophen. DAD is the most severe type of drug-induced lung injury, requiring high-dose steroid therapy early after diagnosis.14 However, there are many unresponsive cases to treatment, and the prognosis of patients with DAD is poor. Our patient was diagnosed with DAD based on CT and pathological findings and was refractory to various drug treatments.
In conclusion, there are cases of acetaminophen-induced SJS in which lung injury progresses rapidly and irreversibly, leading to fatal outcomes.
AUTHOR CONTRIBUTIONS
R. N. participated in the management of the patient, obtained clinical data, drafted the initial manuscript, and revised the manuscript. F. O. helped in conceptualizing and drafting the manuscript. T. C., T. J., and M. E. critically reviewed and revised the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and Declaration (as revised in 2013). Written informed consent was obtained from the patient.
CONSENT
Written informed consent was obtained from the parent of patient to publish this report in accordance with the journal's patient consent policy as the patient is under the age of 16.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.