Autologous blood pleurodesis for surgical pneumothorax and outcome with multimodal cancer treatment in a dog with primary pulmonary mast cell tumor
Abstract
A dog underwent lung lobectomy for removal of a mass. Histopathology was consistent with narrow resection of a mast cell tumor. Postoperative pneumothorax was successfully treated using autologous blood pleurodesis. Progression of disease despite adjunctive treatment with several chemotherapetutic agents and radiation therapy resulted in euthanasia approximately 4 months postoperatively.
1 INTRODUCTION
Mast cell tumors (MCTs) represent the most common type of cutaneous malignancy in dogs; however, primary MCTs localized to the lungs are extremely rare.1, 2 MCTs of visceral origin, including gastrointestinal, spleen, and liver, have been diagnosed in dogs but are minimally characterized and have a generally poor prognosis.3, 4 Metastatic dissemination of MCTs is commonly seen to local lymph nodes, liver, and spleen, while thoracic metastasis is very atypical.5 Primary treatment for MCTs depends on prognostic factors such as histologic grade (applicable to cutaneous MCTs) and stage, but commonly involves surgical excision, chemotherapy, and radiation therapy.3-11 There are only four previously reported cases of canine primary pulmonary MCTs, all of which were euthanized without aggressive treatment with a maximum survival of 3 months.1, 2
Surgical treatment for pulmonary tumors includes partial and complete lung lobectomy via an open or thoracoscopic approach and closure of the affected bronchus via suture ligation or automatic stapling equipment.12-14 Pneumothorax is a reported complication that can arise following lung lobectomy.15 While vacuum-assisted thoracic drainage systems are often successful, there are multiple potential sources of vacuum system failure including air leakage around the thoracostomy tube, tube displacement, or failure of the pulmonary parenchyma to seal.14 Autologous blood patch pleurodesis (ABP), or infusion of autologous blood into the thoracic cavity with the goal of sealing the leak site, is a well-established, low-cost, and effective treatment in humans with persistent pneumothorax following lung lobectomy.16-18 There are reports of ABP use in dogs and cats for correction of spontaneous pneumothorax as well as persistent pneumothorax of various etiologies including postoperative correction of traumatic diaphragmatic hernia.19-21 To the authors' knowledge, there is no report of successful ABP following pneumothorax as a complication of lung lobectomy in veterinary medicine. The following report the details and outcome of a case of presumed primary pulmonary MCT that was treated with surgery, chemotherapy, and radiation therapy as well as successful resolution of pneumothorax following lung lobectomy using autologous blood pleurodesis (ABP).
2 CASE REPORT
2.1 Case history and diagnostics
A 2.5-year-old, 36.3 kg, male castrated boxer was evaluated at a referral veterinary center for a 2 day history of coughing, lethargy, and decreased appetite. Physical examination revealed increased bronchovesicular sounds on thoracic auscultation but no other abnormalities. A complete blood count (CBC) showed a leukocytosis of 26,100 cells/μl (reference range: 6000–17,000 cell/μl) characterized by an eosinophilia of 14,090 cells/μl (reference range: 100–1000 cell/μl). The remaining values on CBC and a venous blood gas were unremarkable. Thoracic radiographs were interpreted by a board-certified radiologist and were reported to show a mixed pulmonary pattern characterized by a consolidated alveolar pattern in the right caudal lung lobe with concern for two soft tissue opaque masses and a multifocal unstructured interstitial pattern coalescing to a patchy alveolar pattern in the left cranial and caudal lung lobes (Figure 1). Differentials based on radiographs included eosinophilic granuloma, parasitic or fungal pneumonia, or neoplasia. Bronchoalveolar lavage performed in the right middle, right caudal, and left caudal lung lobes was consistent with marked eosinophilic inflammation. A mycoplasma PCR and aerobic culture of the lavage fluid were negative. Percutaneous transthoracic fine needle aspirate of the right caudal masses was inconclusive due to low cellularity. The dog was treated for presumptive eosinophilic bronchopneumopathy and was prescribed fenbendazole (50 mg/kg orally [PO] once daily [SID]), prednisone (40 mg/m2 PO SID), and amoxicillin/clavulanic acid (16.5 mg/kg PO twice daily [BID]). Cetirizine (0.45 mg/kg PO SID) was prescribed when minimal improvement in the radiographic pulmonary pattern was noted at 1 month follow-up.
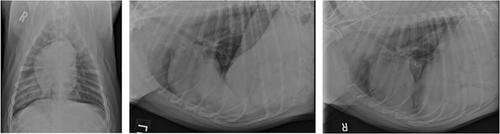
Repeat thoracic radiographs at 2 months following initial presentation noted an additional, well-defined, 4.3 cm diameter mass in the left caudal lung lobe with some resolution of the previously noted masses in the right caudal lung lobe (Figure 2). Computed tomography (CT) of the thorax confirmed the presence of a soft tissue attenuating, contrast enhancing mass in the left caudal lung lobe measuring 6 × 6.5 × 6 cm (Figure 3). An additional soft tissue attenuating nodule was identified in the left caudal lung lobe measuring 1.6 mm, along with tracheobronchial and cranial mediastinal lymphadenopathy. The previously noted right caudal lung lobe masses were not noted on CT. Based on the anatomic location of the nodules and clinical progression, it was determined that the dog was a candidate for left caudal lung lobectomy.
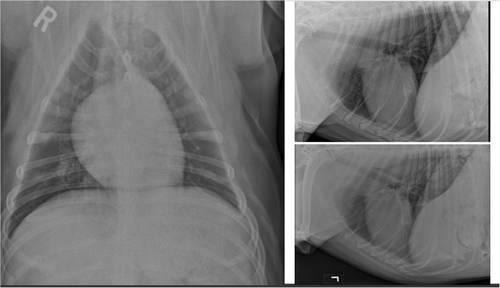
2.2 Surgical treatment
The dog was sedated with dexmedetomidine (3 μg/kg intravenously [IV]), and general anesthesia was induced using ketamine (1.7 mg/kg IV) and propofol (1.4 mg/kg IV). A cuffed endotracheal tube was placed, and anesthesia was maintained throughout the procedure using isoflurane in oxygen at the discretion of the attending board-certified anesthesiologist. Cefazolin (22 mg/kg IV) was administered 30 min prior to the surgical incision and again every 90 min intraoperatively. A left lateral fifth intercostal thoracotomy was performed routinely.14A large intraparenchymal mass occupying the majority of the left caudal lung lobe and severely enlarged tracheobronchial lymph nodes were noted on left thoracic exploration. The lung lobe was isolated and a 30 mm thoracoabdominal stapler with a V3 cartridge (DST Series™ TA™; Medtronic) was placed and discharged across the base of the left caudal lung lobe as close to the hilus as possible. After the lung was transected and the stapler was released, mild hilar hemorrhage was appreciated. Stainless steel hemoclips (Rica Surgical Products) were used to achieve hemostasis. The surgical site was oversewn in an interrupted horizontal mattress pattern with 4-0 polydioxyanone (PDS-II; Johnson & Johnson Medical), and a transfixation ligature was placed across the bronchus. No leaks were appreciated when the chest cavity was filled with warm sterile saline. A 20 French polyvinyl chloride chest tube (Cardinal Health) was placed at the level of the seventh intercostal space, and liposomal encapsulated bupivacaine (Nocita™; Elanco Animal Health) (4.5 mg/kg) was infused into the subcutaneous tissue and skin. The dog recovered uneventfully from anesthesia and was maintained on fentanyl (4 μg/kg/h IV) postoperatively for analgesia. The lung lobe with mass was submitted for histopathologic evaluation, which revealed very narrow (<1 mm) excision of a malignant MCT
2.3 Postoperative progression and autologous blood pleurodesis
Twelve hours postoperatively, a significant increase in respiratory effort was noted and pneumothorax was confirmed via aspiration of the thoracostomy tube. The dog was placed on a continuous underwater thoracic drainage system (PleurEvac; Teleflex) connected to the chest tube. Two days following the lobectomy procedure, the pneumothorax persisted when air was not actively evacuated and an ABP was performed using between 5 and 10 ml/kg as previously described.19-21 The dog was sedated with 0.2 mg/kg methadone IV, and a total of 310 ml of blood (8.5 ml/kg) was drawn from the jugular vein using a 60 ml syringe and administered in six 50-60 ml boluses via the thoracostomy tube into the thoracic cavity and flushed with 5 ml of sterile saline after each bolus. The dog was then placed in right lateral recumbency for 1 h to allow for clot formation under manual restraint. Following ABP, there was no clinically significant pneumothorax based on aspiration of the chest tube every 6 h. The dog was discharged 6 days postoperatively on prednisone (0.25 mg/kg PO SID), trazodone (5 mg/kg PO every 8 h as needed [TID PRN]), gabapentin (14 mg/kg PO TID prn), omeprazole (1 mg/kg PO BID), diphenhydramine (2.4 mg/kg PO BID), amoxicillin/clavulonic acid (18.7 mg/kg PO BID), and enrofloxacin (10 mg/kg PO SID)
2.4 Adjunctive treatment
Two weeks postoperatively, the dog was rechecked for incisional healing and initiation of adjunctive treatment. The incision was well healed, abdominal ultrasound did not reveal any significant abnormalities, and cytology of the liver and spleen did not reveal neoplastic mast cell infiltration. The dog received weekly vinblastine treatments for 3 weeks (2.25 mg/m2 IV) and then two additional vinblastine treatments dosed every 14 days. He was started on a tapering course of prednisone PO (starting at 2 mg/kg/day) and famotidine (2 mg/kg PO BID). Four weeks postoperatively, CBC showed marked eosinophilia of 52,800 cells/μl, and an increased soft tissue opacity was noted in the region of the tracheobronchial lymph nodes and the right middle lung lobe on thoracic radiographs. Toceranib phosphate (Palladia) (2.37 mg/m2 PO) was prescribed on a Monday–Wednesday–Friday schedule. At the subsequent evaluation, the dog's eosinophil count had substantially improved to 13,500 cells/μl, and he was clinically improved at home for 2 weeks following this treatment. At 10 weeks postoperatively, the disease was determined to be progressive and resistant to the performed therapies based on persistent tracheobronchial lymphadenomegaly, progressive nodular pattern within the right middle lung lobe on thoracic radiographs, and resurgence of eosinophilia (14,470 cells/μl) (Figure 4). Due to the apparent progressive disease, vinblastine and toceranib phosphate were discontinued, and lomustine (58.3 mg/m2 PO) was administered. Prednisone was also discontinued due to concern for iatrogenic Cushings disease given the dog's development of a pot-bellied appearance and elevated liver enzymes.
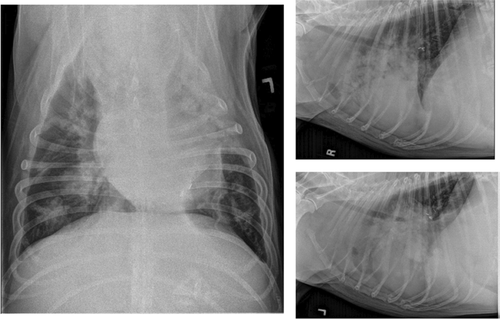
Two weeks (12 weeks postoperative) following initiation of lomustine therapy, the dog was hospitalized for 3 days for respiratory distress. Another dose of lomustine was administered, and outpatient palliative radiation therapy was initiated at that time. The dog received a total of four radiation therapy treatments (400 cGy/treatment) over 3 weeks and had mild clinical improvement following the first three treatments. Four months following surgery and 3 weeks following initiation of radiation therapy, the dog went into severe respiratory distress and humane euthanasia was performed using pentobarbital (86 mg/kg) IV.
2.5 Necropsy examination
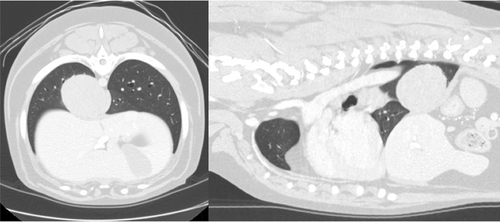
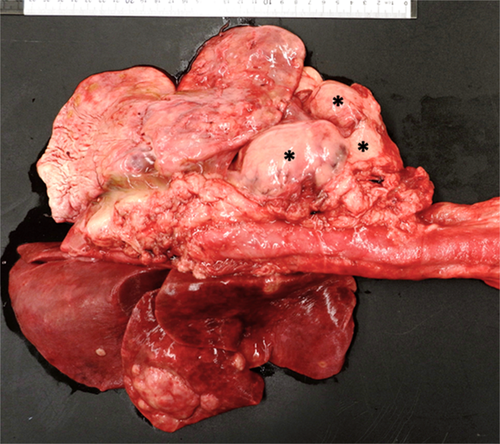
Necropsy revealed an extensive multinodular agglomerate of white, firm, tan, homogenous masses measuring 11 × 10 × 5 cm in the cranial mediastinum causing caudal displacement of the lung fields (Figure 5). Similar nodules were multifocally distributed throughout the lung with the largest mass in the right middle lung lobe measuring approximately 4 cm in diameter. Metastatic invasion of neoplastic cells was noted in the liver and central nervous system. Histopathology of the masses identified pleomorphic neoplastic round cells and robust eosinophilic infiltrates on a fine fibrovascular stroma. Mast cell tumor and lymphomatoid granulomatosis (angiocentric lymphoma) were considered as differentials and further characterization was pursued via special stains, immunohistochemistry, and consultations with the Joint Pathology Center (Silver Spring, MD). Based on results of special staining, lymphomatoid granulomatosis could not be definitively ruled out; however, a consensus among multiple pathologists was for MCT arising from the lung with metastasis to the mediastinum, liver, and central nervous system.
3 DISCUSSION
This case supports that canine primary pulmonary MCTs carry a poor prognosis even with aggressive, multimodal treatment. Canine pulmonary MCTs are uncommon, with only four cases previously reported in the literature, all of which were euthanized within 3 months of diagnosis without extensive treatment.1, 2 In the first case, euthanasia was elected immediately following diagnosis.1 In the second case, surgical lobectomy was attempted but intraoperative euthanasia was ultimately performed based on poor ventilation and hypotension under anesthesia.1 The final two cases report dogs with primary pulmonary masses that were cytologically and histopathologically (via thoracoscopic biopsy) confirmed as MCTs. Both dogs were treated medically with toceranib phosphate without any surgical intervention.2 In both cases, radiographic progression of disease was indicative of a lack of therapeutic response (41 and 56 days), and the dogs were euthanized without further treatment.
The dog in our study was treated with aggressive, multimodal chemotherapy, including lomustine, vinblastine, prednisone, and toceranib phosphate. Combinations of these agents have been successful in both cutaneous MCTs and pulmonary tumors.6-8 Chemotherapeutic treatment with a combination of lomustine and vinblastine for cutaneous canine MCTs had a 57% response rate in one study with a median progression free interval of 30 weeks.8 Combination therapy with vinblastine, prednisolone, and toceranib phosphate has been shown to have a 90% response rate in cutaneous Patnaik grade II and III MCTs.6 Successful use of radiation therapy, both definitive and palliative intent, for MCTs has been reported in the veterinary literature.9-11 Unfortunately, the dog in this study did not show a long duration of response to chemotherapeutic and/or radiation treatments, indicating that canine pulmonary MCT disease may not respond to conventional or established medical therapy as effectively as cutaneous MCT disease.
The dog in this study was also treated successfully with ABP for pneumothorax following stapled lung lobectomy with a good short-term outcome. Two theories for the mechanism of ABP have been postulated. It has been suggested that the coagulated blood adheres to the lung as a patch and seals the air leak directly, or that autologous blood causes inflammation and subsequent adhesions between the lung and pleura resulting in a seal.17 One study suggests that the latter mechanism is less likely as there was no gross evidence of pleural adhesions after 30 days in a rabbit model.22 In this study, the histologic findings were consistent with pleurodesis, but gross pleurodesis was not noted and it was suggested that an impractical length of time would be required to achieve clinical adhesions. However, only 1 ml/kg autologous blood was used, which is markedly reduced compared to other reports of ABP in non-human species, which may have decreased the subsequent inflammatory response. The amount of intrapleural instilled autologous blood is between 5 and 10 ml/kg when the technique has been previously described in dogs, and the use of the ABP terminology is consistent with previous reports in the veterinary literature.19-21 However, intrapleural instillation of autologous blood may be a more physiologically accurate term.
In humans, it has been demonstrated that ABP is a highly effective and safe procedure that can successfully correct pneumothorax following surgical lobectomy.15-18 Multiple techniques have been reported in the literature for ABP in humans; most studies describe the use of 50–120 ml of autologous blood retrieved and immediately inserted into an apical chest tube without anticoagulant. Andretti et al. found an increased efficacy of ABP when 100 ml blood was used compared to 50 ml.18 This same report administered sterile saline as flush following blood pleurodesis, similar to the technique in this case, where other reports did not use saline. Patients are reportedly asked to rotate, though timing varied from constant rotation for 40 min, to every 15 min for 2–6 h.16-18
In addition to eliminating air leaks, ABP has been associated with earlier chest drain removal and shorter duration of hospital stay.17 A review of pneumothorax by Pawloski et al.15 concluded that ABP should be the gold standard of postoperative care in human patients. In canine patients, blood pleurodesis has been reported effective for correction of pneumothorax following surgical correction of a traumatic diaphragmatic hernia, as well as spontaneous and persistent pneumothorax of multiple etiologies.19-21 To the author's knowledge, this is the first reported use of ABP in veterinary patients for complications following lung lobectomy. The case in this report suggests that, similar to human medicine, use of ABP in canines to treat pneumothorax secondary to surgical lobectomy would be successful. However, further evaluation with larger sample size is necessary before any conclusions or recommendations can be made.
This case demonstrates further that primary pulmonary round cell tumors that resemble MCTs are very rare in canine patients and likely carry a grave prognosis, similar to visceral MCTs. The patient in this study lived approximately 4 months following histopathologic diagnosis, presumably due to aggressive treatment with surgical excision of the tumor, chemotherapy, and radiation therapy. This survival time surpasses the longest reported survival for pulmonary MCT, though, the response to treatment was not substantial.1, 2
AUTHOR CONTRIBUTIONS
Daniel Shinsako: Contributed to the acquisition of data and manuscript preparation and review. Alison R. Masyr: Contributed to acquisition and interpretation and of data and manuscript preparation and review. Miranda Vieson: Contributed to acquisition of data, histopathologic assessment, and manuscript preparation and review. Hadley E. Gleason: Contributed to case study conception, acquisition of data, and manuscript preparation and review.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST
There are no financial or other conflicts of interest related to this study.
ETHICAL APPROVAL
This retrospective case study was performed in full compliance with research ethics norms.
CONSENT
Written informed consent was obtained from the client to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




