COVID-19 vaccination and alopecia areata: a case report and literature review
Abstract
Coronavirus disease 2019 (COVID-19) is a major concern worldwide and various vaccines have been developed and approved for it, however some immune-related issues of COVID-19 vaccines should be considered and individualized for patients. In this study we report two cases of rapidly progressive hair loss following COVID-19 vaccination.
1 INTRODUCTION
Over 468 million infections and more than 6 million deaths worldwide have been caused by the Coronavirus disease 2019 (COVID-19).1 Various vaccines have been developed and approved for COVID-19 globally. Due to the new variants (e.g., Omicron), the use of additional doses is also being reviewed and approved for prevention.2 There is still insufficient information on the immune-related issues of COVID-19 vaccines and whether or not they can cause the flare of autoimmune diseases.3 This study reports on two patients who developed rapidly progressive hair loss following COVID-19 vaccination.
2 CASE PRESENTATION
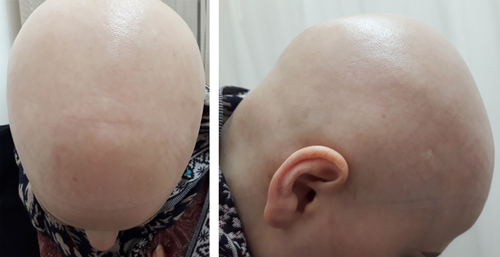
A 23-year-old woman presented to the dermatology clinic with complaint of local hair loss on her scalp a week after receiving the first dose of Oxford/AstraZeneca COVID-19 vaccine (AZD1222) in August 2021. She also reported myalgia following the vaccination, which was relieved by acetaminophen. She had no past medical or family history of alopecia areata (AA) and had not received any medications. A positive pull test demonstrated oval-shaped patchy hair loss with defined borders and no scarring or scaling. Her KOH test was negative. Treatment with betamethasone cream 0.1% and pimecrolimus cream 1% started immediately for her after the diagnosis of AA. After 3 weeks, the patient returned with total hair loss on the scalp and eyebrows (Figure 1). Laboratory tests including COVID-19 and antinuclear antibody test, CBC, and liver, kidney, and thyroid functions were all normal with the exception of elevated anti-thyroid peroxidase (anti-TPO). Our diagnosis was confirmed when the biopsy revealed peribulbar lymphocyte infiltration with increased miniaturized hairs. Treatment with systemic corticosteroid (oral prednisolone, 300 mg monthly, for 3 months) was started for improvement. We asked the patient to return after hair regrowth to taper her medication, and also offered her tofacitinib (a systemic JAK/STAT pathway inhibitor) as another treatment option, which was refused by the patient given its high costs in our country.
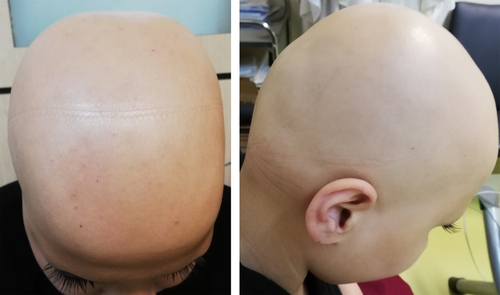
The second case, a 26-year-old woman with a history of AA relieved by an intralesional corticosteroid, presented with complaint of generalized hair loss on the scalp 2 weeks after receiving the second dose of AZD1222 in January 2021. The condition had started with patchy alopecia, and over the course of a month, it had evolved to diffuse hair loss. She reported infection with COVID-19 1 year before.
A physical examination revealed diffuse hair loss only throughout the scalp, with no scarring or scaling (Figure 2). Laboratory tests were normal except for increased anti-TPO.
A diagnosis of alopecia totalis was made for her based on her history and physical characteristics, and treatment with systemic corticosteroid was initiated (oral prednisolone, 300 mg monthly, for 3 months). The patient was asked to return after hair regrowth to taper her medication. The other treatment option was tofacitinib, which was again refused by this patient for the same reason as the first case.
3 DISCUSSION
The SARS-CoV-2 virus can make the immune system overactive due to the interaction and molecular mimicry between the virus and self-antigens. Similar to the disease itself, COVID-19 vaccines can increase inflammatory responses.4 NF-kB activation, which leads to the release of many cytokines, and elevated IFN and IL-6, are linked to AA pathology and might cause hair loss.5 AZD1222 expresses the S glycoprotein, which stimulates both antibody production and Th1 cell activation.4 This type of hair loss has also been noticed among recipients of other types of COVID-19 vaccines, such as BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna)6 (Table 1). There has been a history of AA in the majority of the cases reported in the literature3, 6, 7 but some of the reported cases6, 8 and one case in our study have no personal or family history of AA. Vaccines have been suggested as potential triggers for autoimmune diseases, mostly in genetically susceptible individuals, and have also been proposed as a probable cause of AA. Herpes zoster, hepatitis B, Japanese encephalitis, influenza, and quadrivalent HPV (HPV 6, 11, 16, and 18). COVID-19 vaccines have all been linked to an increased incidence of AA in studies.3, 4, 7 Thyroid dysfunction is more common in individuals with AA than in the general population, implying that thyroid function and autoantibodies should be screened more frequently in these patients.9 According to Naranjo algorithm, the occurrence of this complication is classified as a probable complication.10
| Case | Sex | Age | Vaccine type | PMH | Flare time after vaccination | Severity of hair loss | Time | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 32 | AstraZeneca | AA | Few days | Patch | June 2021 | 6 |
| 2 | F | 76 | Pfizer | AA | After 2 weeks of first dose | Patch | March 2021 | 3 |
| 3 | F | 59 | AstraZeneca | AA/autoimmune thyroiditis | 3 weeks after the vaccine | Patch | March 2021 | 3 |
| 4 | F | 29 | AstraZeneca | AA | 2 weeks after first dose | Patch | March 2021 | 3 |
| 5 | M | 80 | Pfizer | NA | 1 weeks after first dose | Totalis | Not reported | 7 |
| 6 | F | 33 | Moderna | Steatosis, chronic hepatitis B | 2 months after 2nd dose | Patch | March 2021 | 5 |
| 7 | F | 57 | Pfizer | AA | 4 months after 2nd dose | Totalis | February 2021 | 5 |
| 8 | F | 62 | Moderna | AA | 2 months after 2nd dose | universalis | June 2021 | 5 |
| 9 | F | 28 | Pfizer | AA, Hashimoto thyroiditis | A week after 2nd dose | universalis | June 2021 | 5 |
| 10 | F | 29 | Pfizer | Elevated levels of TgAb and TPOAb | 1 week after 2nd dose | Patch | April 2021 | 5 |
| 11 | M | 22 | Moderna | Elevated TgAb | 1 month after 2nd dose | Patch | April 2021 | 5 |
| 12 | M | 15 | Pfizer | NA | Within 1 week after 2nd dose | Patch | June 2021 | 5 |
| 13 | M | 61 | Pfizer | Joint pain | 2 weeks after 1st dose | Totalis | March 2021 | 5 |
| 14 | M | 16 | Pfizer | NA | Within 1–2 weeks after 1st dose | Patch | June 2021 | 5 |
| 15 | F | 23 | AstraZeneca | Elevated TPOAb | 1 week after first dose | Totalis | August 2021 | Present study |
| 16 | F | 26 | AstraZeneca | AA/elevated TPOAb | 2 weeks after second dose | Totalis | January 2021 | Present study |
- Abbreviations: AA, alopecia areata; NA, not applicable; TgAb, thyroglobulin autoantibody; TPOAb, thyroid peroxidase antibody.
Generally, there are few reports around the world about hair loss following COVID-19 vaccination, and most of the reported cases have had a previous history of the disease, and in some cases, the anti-TPO test has also been positive. To conclude, our cases and previous reported cases suggest that COVID-19 vaccines may have a role in inducing AA in genetically predisposed patients, especially those with positive anti TPO; however, further studies are required on the subject.
AUTHOR CONTRIBUTIONS
Dr. Zakiye Ganjei performed patient management, follow-up, and provided photographs. Dr. Mohammad Yazdan Panah, Dr. Rahem Rahmati, and Dr. Fatemeh Zari Meidani were drafting the manuscript and reviewed the literature. Dr. Azam Mosavi was responsible for revising the manuscript and also follow-up of the patient.
ACKNOWLEDGMENT
None.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
ETHICAL APPROVAL
The authors declare human ethics approval was not needed for this study.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.