Monitoring of hemodynamic changes during huge cystic lymphangioma resection in a 91-day-old infant
Abstract
Cystic lymphangioma (CL) is a rare benign lesion that can be treated with surgical resection. We describe the monitoring of hemodynamic changes in a 91-day-old girl undergoing resection of a huge CL. Monitoring was managed through continuous central venous pressure monitoring using a central catheter in the inferior vena cava.
1 INTRODUCTION
Cystic lymphangioma (CL) is a benign lesion caused by malformation of the lymphatic system; it has an incidence of 1 in 2,000–4,000 people. It is usually detected not only during the neonatal or infancy period but can also be seen in adulthood. CL occurs mainly in the neck and axilla, but rarely in the abdominal wall, inguinal region, buttocks, anogenital region, retroperitoneal areas, tongue, parotid, mediastinum, spleen, prostate, or other regions.1 Most CLs are asymptomatic; however, airway obstruction, feeding problems due to dysphagia, infection, and intracystic hemorrhage might occur depending on the location.2
Diagnosis can be made on prenatal sonography, and computer tomography or magnetic resonance imaging (MRI) can be used after birth.1 Surgical resection is considered to be the preferred treatment; however, other methods such as aspiration and sclerotherapy have also been used.3 Radio-frequency ablation and laser excision have been the more recent treatment options.3 When surgical treatment is required, there are various anesthetic considerations depending on the location, extent, and size of the CL.
In this case, a huge CL of 18 cm × 13 cm or more in size invaded a very wide area, including the trachea, brachial plexus, and vertebra. The size of the CL was also very large compared with the patient's volume. The duration of surgery for a large CL could be very long, and there is a risk of bleeding during resection; hence, intraoperative hemodynamic changes were expected. We present this case in which the monitoring of hemodynamic changes was successfully performed through continuous monitoring of central venous pressure (CVP).
2 CASE REPORT
The patient was a 91-day-old female infant with a height of 65 cm and a weight of 5.3 kg. A detailed fetal anomaly ultrasound scan performed at the gestational age (GA) of approximately 17 weeks showed a multicystic mass in the right neck, right shoulder, and right axillary areas; no other anomalies were found. Due to the large cystic mass, she was born with a weight of 3.4 kg through a cesarean section at 37 weeks of GA in the previous medical center. At birth, there were no abnormalities except the large cystic mass. Due to the cystic mass, there was frequent dyspnea and low oxygen saturation, and she refused feeds. After 1 month of age, sclerotherapy was performed using doxycycline, and tracheostomy was performed at the previous medical center to secure the airway. Despite sclerotherapy, the size of the cystic mass did not decrease significantly, and feeding was inadequate.
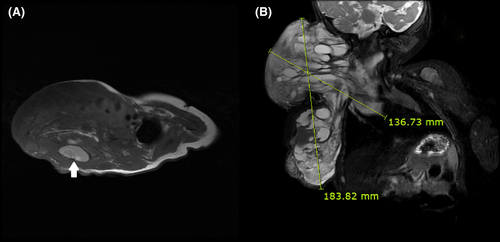
Subsequently, she was transferred to our hospital for surgical treatment. Laboratory examination, MRI, and ultrasound were performed for preoperative evaluation. The MRI scan showed that the size of the cystic mass had increased to 18 cm × 13 cm or more, and it extended from behind the trachea on the upper side to the back side thoracic vertebra area on the lower side. The image also revealed that the CL was multicystic and had an intracystic hemorrhage (Figure 1). The results of the laboratory examination showed no abnormal findings, and the hemoglobin (Hb) level on a complete blood count test was determined to be 9.4 g/dL. The airway was maintained with a tracheostomy, and tubal feeding was performed for proper feeding.
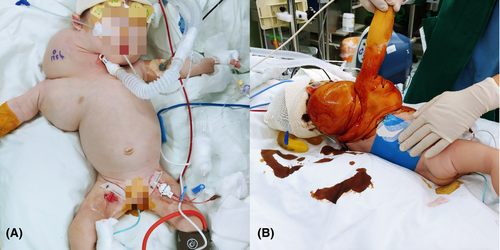
For anesthesia, induction and maintenance anesthesia was performed using sevoflurane through the tracheostomy site, and 3 mg of rocuronium was administered intravenously. A 4-Fr bilumen central catheter (CareflowTM Central Venous Catheter Kit Seldinger Technique, Merit Medical Singapore Pte. Ltd.) was inserted into the left femoral vein under ultrasonographic guidance, a 22 G arterial catheter (ARROW R Arterial Catheterization Set, Teleflex Inc.) was placed in the right femoral artery, and a urinary catheter was inserted. Arterial blood gas analysis (ABGA) was performed at intervals of 1 h to 1 h and 30 min. In the initial ABGA, the Hb level was measured to be 8.2 g/dL. Continuous CVP monitoring was performed with the distal end of the central catheter inserted through the left femoral vein, and continuous arterial blood pressure was measured using the right femoral arterial catheter. We confirmed the position of the catheters using fluoroscopy. The patient was placed in a left lateral position, and her body temperature was maintained using a forced-air warming blanket (Figure 2). Hartmann's solution was used as intraoperative crystalloid.
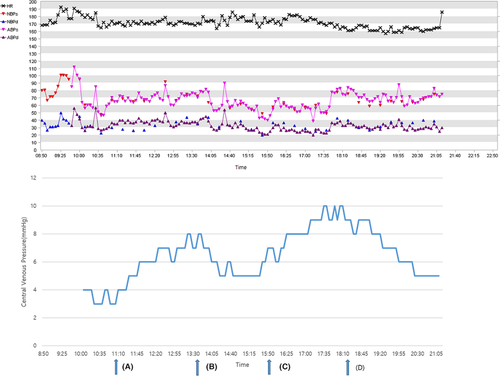
Fluid resuscitation and transfusion were considered based on the intraoperative CVP values, Hb levels on ABGA, arterial blood pressure (ABP), and heart rate (HR). In particular, the threshold of transfusion was set at 7.0 g/dL, but transfusion was determined by comprehensively considering CVP, ABP, and HR.4 After 1 h and 25 min of induction, the systolic ABP decreased to less than 60 mmHg, and 10 µg of phenylephrine was administered. Subsequently, the ABP increased to more than 80 mmHg; however, the CVP value decreased from 4 mmHg to 3 mmHg. At that time, the Hb level on ABGA was found to be 6.8 g/dL, and blood transfusion was initiated. Seventy mL of red blood cells (RBCs) were transfused for 2 h and 30 min using an infusion pump. After the transfusion, the CVP increased to 8 mmHg, ABP was maintained at 60–90 mmHg, and Hb was 7.8 g/dL. Subsequently, the ABP and HR were continuously stable, and Hb was maintained at 8.8 g/dL. Seven hours after induction, the patient's ABP dropped to 40 mmHg, and 10 µg of phenylephrine was administered. The ABP recovered to about 70 mmHg; however, the CVP decreased from 8 mmHg to 5–6 mmHg, and Hb was 7.0 g/dL on ABGA. Transfusion was initiated again, and 50 mL of RBCs was transfused over 2 h. As a result, the CVP increased to 9–10 mmHg, and the ABP was maintained at 60–70 mmHg. The V/S remained stable, the CVP was maintained above 5 mmHg, and Hb on ABGA was 8.8 g/dL (Figure 3).
For the surgery, a skin incision was made on the neck and axilla on the mass, and an excision was made along the skin flap. Most of the mass was resected except for a part of the mass surrounding the brachial plexus in the axillary area and a part of the mass that connected to the mediastinum below the clavicle. During surgery, the vagus nerve, brachial plexus, cervical branch of the facial nerve, and phrenic nerve were preserved, and there was no vessel scarification. The surgery was completed without any major complications, and the total duration of the surgery was 12 h and 27 min. The total amount of crystalloid administered was 599 mL, and 120 mL of RBCs was infused; the estimated blood loss was 100 mL and the urine output was 100 mL. The patient was transferred to the intensive care unit with postoperative sedation.
3 DISCUSSION
There are various anesthetic considerations during surgery for CL, depending on its location and size. In the current case, the size of the CL was 18 cm × 13 cm or more, and its extent was very wide. As the CL extended posterior to the trachea on the medial side, respiratory distress due to airway compromise was expected. In such cases, a thorough preoperative evaluation of the airway is essential to secure the airway, and readiness for the “Cannot Intubate, Cannot Ventilate” situation is critical.5 However, in this case, tracheostomy was performed at the previous medical center due to airway distress caused by the very large tumor size; therefore, securing the airway was not difficult.
Another important anesthetic consideration is monitoring of hemodynamic changes and fluid management. The CL is usually large and extensive and involves various muscles, nerves, and vessels. In this case, especially since the patient is an infant, the proportion of CL in the body is very large. Therefore, it is very difficult to surgically remove the CL while minimizing damage to the surrounding tissues; hence, the duration of the surgery might be prolonged. The occurrence of hypovolemia due to loss of body fluid or bleeding should be expected. Adequate fluid therapy can prevent organ failure and reduce mortality; it is important for maintaining cardiovascular function; therefore, intraoperative monitoring of circulation volume is important.6 There are several methods for monitoring the circulating volume. Cardiac output (CO), cardiac index, and volume status can be measured using pulse index contour CO. This method is very accurate, and an accurate volume status of the patient can be obtained through the global end-diastolic index; however, it is an invasive method,6 and it was difficult to use because the device was not available at this center. Alternatively, the FloTrac system can be used to measure CO, systemic vascular resistance, and stroke volume variation (SVV). However, it is not recommended for use in infants because of the concerns about accuracy.7 A relatively recent method is noninvasive cardiac output monitoring. It can measure CO and SVV by analyzing the variation in the oscillating current of the thoracic cavity using four electrodes. However, it is important to place the four electrodes in the correct position, which is not easy in infants and might lead to inaccurate values.8 Moreover, in this patient, it was impossible to attach the electrodes due to the large anatomical area involved in the surgery.
Hence, we performed continuous CVP measurements to monitor the patient's volume status.
For CVP measurement, the catheter is usually placed inside the superior vena cava (SVC) in the thoracic cavity, and an internal jugular or subclavian venous approach is used. However, there is a risk of pneumothorax or chylothorax. In this patient, the CL extended from the right anterior chest wall to the posterior vertebral area; hence, access to the right internal jugular vein was difficult. Therefore, we placed the catheter in the inferior vena cava (IVC) through the left femoral vein. Although the level at which the catheter tip should be located is not clearly defined, it is considered appropriate to be located at the entry point of the renal vein.9 So, we verified the location of the catheter tip using fluoroscopy and confirmed that it was positioned appropriately. The IVC is located intra-abdominally, and the CVP thus measured might have a different value compared with that at the intrathoracic site. However, since the pressures of IVC and SVC are similar in children undergoing mechanical ventilation, anesthetic management was successfully performed by placing a catheter in the IVC to maintain an appropriate CVP value.10 Nevertheless, previous studies have shown that CVP has a poor correlation with intravascular volume status or circulating blood volume.11, 12 Moreover, CVP is determined by cardiac function and venous return, and various factors play a role in the CVP level. However, CVP is essential to maintain cardiovascular function. In situations where it is difficult to measure the factors affecting CVP and no significant change in such factors is expected, the CVP is still useful.13 This is especially true in situations where other methods for monitoring of hemodynamic change are difficult to use such as in the case presented here. Additionally, CVP monitoring was possible through a femoral catheter in the intensive care unit after surgery and during surgery.
In recent times, various devices have been developed to measure cardiovascular function. However, each device has clear indications and limitations. It is also difficult to install all of the devices in a specific medical center. This case report demonstrated successful anesthetic management through femoral venous catheterization in challenging surgical or anesthetic situations due to a large CL in an infant, wherein it could be difficult to use the devices to monitor cardiac function.
ACKNOWLEDGEMENTS
This study was supported by research funds from Dong-A University.
CONFLICTS OF INTERESTS
The author reports no conflicts of interest in this work.
AUTHOR CONTRIBUTIONS
Sung Wan Kim designed this case report. Kyung Hyun Lee wrote the manuscript. Deuk Won Eom coauthored the manuscript and searched literature. Tae Hyung Kim coauthored the manuscript. Sang Yoong Park provided medical consultation and reviewed the manuscript.
CONSENT
Written consent was obtained from parents to use data and photographs and publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.