Targeting colorectal cancer using dietary flavonols
Abstract
Colorectal cancer is among the well-known forms of cancer and a prominent cause of cancer demises worldwide. In vitro experiments reinforced by animal studies, as well as epidemiological studies of human colorectal cancer propose that the growth of this disease can be moderated by eating aspects. Dietary intake including green vegetables and fruits may result in the reduction of colon cancer chances. The finding suggests that the combinations of dietary nutrients may deliver additive or synergistic effects and might be a powerful method to avoid or eradicate colon cancer beginning and/or development. Flavonols are one of the most widespread dietary nutrients of the polyphenols-flavonoids and major constituent of Allium and Brassicaceae vegetables. Flavonols present in vegetables of Allium and Brassicaceae family are kaempferol, myricetin, quercetin, and isorhamnetin. These flavonols are claimed to have antiproliferative activity in vivo and in vitro against colorectal cancer. The objective of this review is to summarize the role of flavonols obtained from dietary sources in the prevention and treatment of colorectal cancer.
Abbreviations
-
- 5-FU
-
- fluorouracil
-
- AIN
-
- American Institute of Nutrition
-
- AMPK
-
- 5′ adenosine monophosphate-activated protein kinase
-
- AOM
-
- azoxymethane
-
- ATM
-
- ataxia telangiectasia mutated
-
- CRC
-
- colorectal cancer
-
- CSK
-
- C-terminal Src kinase
-
- CY
-
- cytochrome
-
- CYP
-
- cytochrome P450
-
- DNA
-
- deoxyribonucleic acid
-
- DSS
-
- dextran sodium sulfate
-
- HCT-15
-
- human colorectal carcinoma
-
- HT-29
-
- human colorectal adenocarcinoma cell line
-
- IC50
-
- half-maximal inhibitory concentration
-
- MAPK
-
- mitogen-activated protein kinase
-
- MCF
-
- Michigan cancer foundation human breast cancer cells
-
- MTT
-
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
-
- PUMA
-
- p53-upregulated modulator of apoptosis
-
- TBARS
-
- thiobarbituric acid reactive substances
-
- TCF
-
- T cell transcription factor
-
- TRAIL
-
- tumor necrosis factor-related apoptosis-inducing ligand
1 INTRODUCTION
Colorectal cancer is one of the most fatal forms of cancer; it is among the commonly diagnosed nature of cancer. A combination of various factors such as the lack of physical exercise, oxidative stress, mutation of oncogenes, environmental factors, dietary factors, and hereditary factors leads to the development of the diseases. A cascade of reaction due to persistent oxidative stress results in DNA damage, which leads to the formation of precancer lesions in mucosal cells and also inhibit the activity of the tumor suppressor gene further resulting in one of the most common and fatal colorectal cancer. Here, antioxidants may play a critical role. Fortunately, a number of food articles are rich sources of such phytochemicals. Recently various in vitro studies and animal experimentation have suggested that dietary factors may play a significant part in the initiation or control of human colorectal cancer. Studies have shown that individuals who ingest regimes rich in phytochemicals particularly polyphenolic compounds with strong antioxidant properties have poorer degrees of colorectal cancer (Figure 1). A repeated acquaintance of colonic cells to these polyphenolic compounds present in the diet not only helps in inhibiting the progress of cancer cells but may be efficacious in preventing colon cancer [1-4].
Flavonoids are widely distributed polyphenolic compounds in nature and a prevalent component of the human diet. Many food flavonoids influence different cellular processes to reduce the changes in colon epithelia, inhibit tumor formation, and act as chemopreventive blocker agents or, chemopreventive suppressor agents using different biological mechanisms of action. However, their exact mechanism of action in vivo is still not established; it may be due to inhibition of angiogenesis, metastasis, initiation of apoptosis, cell cycle arrest, antisurvival, anti-inflammatory, and antiproliferative effects. Flavanoids are supposed to have CYP1 inhibitory action by a significant interaction with cytochrome P450 CYP1 enzymes, thereby resulting in carcinogenesis blockage at the initiation stage. Researchers have tried to derive structure-activity relationships for these potential therapeutic agents which might lead to new drug discovery. Further, as a lot of flavonoids are present in our diet, a greater understanding of their anticancer properties might also help us modify our dietary habits and develop an effective supplement therapy that may provide additive or synergistic effects and could be a potent way to prevent or eliminate colon cancer initiation and/or progression [5-13].
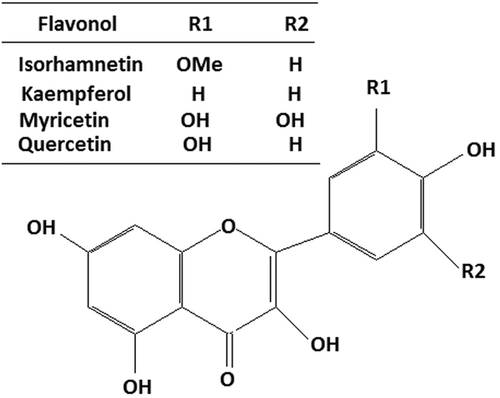
Chemical classification of flavonoids includes isoflavones, flavanones, flavanols, flavonols, flavones, and anthocyanidins. They are abundantly found in Allium and Brassicaceae vegetables. Chive (Allium schoenoprasum L.), leek (Allium porrum L.), shallot (Allium ascalonicum Hort.), scallion (Allium fistulosum L.), and above all garlic and onion are popular and routine parts of dietary intake. Brassicaceae vegetables are also considered an integral part of the human diet chart. Cauliflower, broccoli, brussels sprouts, cabbage, and kale, represent popular B. oleracea, a class of vegetables (Table 1). Various clinical studies have found the reduced risk of colon cancer associated with higher intake of vegetables of these classes. Flavonoids, subclass flavonols, and organosulfur compounds are among the chief chemical constituents present in these vegetables. Kaempferol, myricetin, quercetin, and isorhamnetin are the flavonols present in the above vegetables which have shown some promising in vivo and in vitro antiproliferative action against colorectal cancer (Table 2). This review emphasizes the pronounced prospect of flavonols in cancer prevention and therapy. It is apprehended to give an inclusive summary of the existing knowledge and integral modes of action, concentrating on the flavonols present in the Allium and Brassica family [14-17].
| No | Class | Subclass | Polyphenols | Food source |
|---|---|---|---|---|
| 1 | Phenolic acids | Hydroxy-benzoic acid | Gallic acid | Tea, blackcurrent |
| Protocatechuic acid | Rasberry, mushroom | |||
| Syringic acid | Acai palm | |||
| Hydroxy-cinnamic acid | Ferulic acid | Cereal grains | ||
| Caffeic acid | Kiwi | |||
| Coumaric acid | Plum | |||
| Sinapic acid | Potato, coffee, chicory, pear, flour | |||
| 2 | Flavonoids | Flavonols | Galangin | Lesser galangal (Alpinia officinarum) |
| Kaempferride | Onion, olives, lettuce, parsley | |||
| Kaempferol | Broccoli, leek | |||
| Myricetin | Broccoli, tomato, cherry, blueberry, fruit peels | |||
| Quercetin | Onions, tea, curly kale, bark, and rind of numerous plants | |||
| Flavones | Apigenin | Celery, apple skin | ||
| Bacalein | Roots of Scutellaria baicalensis | |||
| Chrysin | Berries | |||
| Diosmetin | Oregano, spearmint | |||
| Isoflavones | Daidzein | Soy food, soybeans, legumes | ||
| Genistein | Miso | |||
| Flavanones | Eriodictyol | Citrus fruits | ||
| Hesperidin | Lemon juice, citrus fruits | |||
| Naringenin | Citrus fruits | |||
| Silymarin | Coriander seeds, turmeric root | |||
| Flavanols (catechins) | Catechin | Red wine, red grape, tea | ||
| Gallocatechin | Pomegranate, bananas | |||
| Epicatechin | Grapes, peach, apricot, apple, red wine | |||
| Epigallocatechin 3-gallate | Tea | |||
| Anthocyanidins | Cyanidin | Blackberry, berries | ||
| Delphinidin | Black grapes, cherries | |||
| Pelargonidin | Raspberry | |||
| Peonidin | Blueberry, red grapes | |||
| Malvidin | Strawberry, red wine, plum, cherry | |||
| Chalcones | Hop chalcones | Beer, hops | ||
| 3 | Stilbenes | Resveratrol | Grapes | |
| 4 | Lignans | Secoisolariciresinol | Linseed |
| Names of dietary sources | Content (mg/100 g) | |||
|---|---|---|---|---|
| Quercitin | Kaempferol | Myricetin | Isorhamnetin | |
| Onion | 20.30 | 0.65 | 0.03 | 5.01 |
| Garlic | 1.74 | 0.26 | 1.61 | − |
| Leek | 0.09 | 2.67 | 0.22 | − |
| Broccoli | 3.26 | 7.84 | 0.06 | − |
| Cabbage | 0.28 | 0.18 | − | − |
| Cauliflower | 0.54 | 0.36 | 0.00 | − |
2 QUERCETIN
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is the aglycone form of a flavonol, widely distributed in dietary sources. It combines with many flavonoid glycosides such as rutin and quercetin. It is mainly distributed in the perinuclear and nucleoli areas of the cell. It is an antioxidant, like many other phenolic heterocyclic compounds. Quercetin has potential chemopreventive activity. Another observation suggested that the effects of dietary quercetin on colon cancer risk may vary with varying intake of fruit or tea. It was also concluded that quercetin has a defensive effect only on proximal colon cancer [18, 19]. There are several reports on the activity of quercetin in colorectal cancer both in vivo and in vitro [20-27]. Quercetin prevents p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. These effects of quercetin recommend a conceivable chemopreventive role for this molecule in colorectal carcinogenesis. It is reported to initiate autophagy in Ha-RAS-transformed human colon cells by modulating deprivation of oncogenic Ras. Quercetin facilitates frequent mutations of RAS genes by downregulating the concentration of oncogenic RAS genes in affected cells and acts as a chemopreventive agent [20]. Quercetin prevents human DLD-1 colon cancer cell progression and polyamine biosynthesis by affecting the polyamines and ornithine decarboxylase. Both of them are convoluted in cell progress and differentiation. It initiates apoptosis and decreases the polyamine biosynthesis [21]. Quercetin also inhibits human SW480 colon cancer growth in association with the inhibition of cyclin D1 and survivin expression at the molecular level through the Wnt/beta-catenin signaling pathway. The expression of cyclin D(1) and survivin is inhibited via the Wnt/beta-catenin signaling pathway attributing to its antitumor effect [22]. Dietary quercetin modulated exposed confluent Caco-2 monolayers to exhibit a decrease in cell differentiation. The biphasic effect on cell proliferation was also observed in human colon cancer cell line Caco-2 [23]. It was reported that quercetin boosted TRAIL-tempted apoptosis by initiating the redistribution of DR4 and DR5 into lipid rafts. It was projected that quercetin, through its capacity to redistribute death receptors at the cell surface, facilitates death-inducing signaling complex formation, and activation of caspases in response to death receptor stimulation [24].
The treatment of HT-29 colon cancer demonstrated the mechanism of its action. Quercetin was found to activate AMPK to modulate apoptosis via p53-dependent apoptotic cell death leading to a significant reduction in tumor in HT-29 colon cancer cells [25]. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. In vitro studies indicated the cytotoxic nature of quercetin, as it initiated the differentiation in undifferentiated cancer cell lines. It also inhibited the active proliferation of cells [26]. Another study indicated its inhibitory activity over cell viability by inducing mutation in RAS genes. Levels of Ras proteins and half-life of oncogenic Ras were found to be decreased. Autophagic responses were also observed [27]. Another finding suggested the role of quercetin and luteolin in a decrease in drug resistance leading to increased 5-FU effects in CO115 p53 WT tumors using microsatellite instability therapy. The combination therapy increased apoptosis by enhancing p53 expression [28]. The effect of quercetin was also evaluated using Caco-2 cells. It involved the study of its expression over 4000 human genes. It was found to positively influence the activity of tumor suppressor genes using signal transduction pathways such as MAPK and TCF [29]. Sulphated derivative of quercetin, quercetin-5′, 8-disulfonate was found to influence the ROS-dependent apoptosis pathway to inhibit cell proliferation in the S phase. The study involved LoVo cells and MCF-7 cells. The effects were dose-dependent [30]. Another study reported that curcumin and quercetin caused a dose-dependent enhancement of tumors induced by AOM in the colon model, upon azoxymethane-induced colon cancer. A significant decrease in cell viability was evident in a dose-dependent manner. In vivo experiments revealed promising preventive or therapeutic efficacy [31]. Quercetin, was also found to reduce colorectal carcinogenesis of azoxymethane-treated rats in high dosage; however, rutin was not found to be effective possibly due to its low bioavailability [32]. Similarly, azoxymethane-treated mice were kept on a standard AIN-76A diet and investigated for focal areas of dysplasia and cyclin D(1) expression. Quercetin was found to decrease not only the total number of focal areas of dysplasia but also the number of mice exhibiting the same [33, 34]. The synergistic effect of quercetin and kaempferol was evident in studies conducted using HuTu-80, Caco-2, and PMC42. A significant reduction in total cell proliferation was seen possibly owing to reduced nuclear proliferation antigen Ki67 expression [35-37]. In the year 2019, Yang et al. [38] induced apoptosis in colorectal cancer cells via JNK signaling pathways. Recent studies themselves depicted that the use of quercetin restricts colon cancer via modulating antiaging protein expression [39].
3 KAEMPFEROL
Kaempferol is a polyphenol antioxidant (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) under the subclass flavonols, dispersed extensively in Allium and Brassica vegetables and fruits. It is widely distributed in many edible plants. Its daily dietary intake was estimated to be up to approximately 10 mg/day. Kaempferol has established a prodigious deal of consideration in research, due to its certain part in tumbling many diseases such as osteoporosis, diabetes, neurodegenerative diseases, anxiety, infectious diseases, allergies, inflammation, and pain. Various in vitro and in vivo studies have also testified it to display anticancer, anti-inflammatory, anti-allergic, anti-asthmatic, antimicrobial, and antioxidant activities. It is also used in traditional folk medicines. Further, several epidemiological lessons evaluated the positive interaction between the consumption of kaempferol-rich foods and its role in sinking many forms of distortions such as lung, ovarian, gastric, and pancreatic cancers in the human population. Numerous clinical investigations have demonstrated its therapeutic and anticancer effects. Kaempferol can be used as an adjuvant with chemotherapeutic medications in cancer treatment plans to make cells more susceptible to cytotoxicity. Kaempferol scavenges free radicals and inhibits the neoplastic process [40]. A study over male Wistar rats was performed to reveal the effect of kaempferol on tissue lipid peroxidation, its efficacy with irinotecan, and its antioxidant properties against 1,2-dimethyl hydrazine-induced colorectal cancer. Kaempferol was found to lower liver thiobarbituric acid reactive substances level and erythrocyte lysate. It rejuvenated various antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase. The outcomes supported the role of kaempferol as a chemopreventive agent in colorectal cancer [41]. An experiment over human HCT116 colon cancer cells revealed its mechanism of action. It activates caspase-3 cleavage and induces cytochrome c release from mitochondria using Bcl-2 family proteins (i.e., PUMA). It was found to affect the Ataxia-Telangiectasia Mutated-p53 pathway. The p53 upregulated modulator was also involved in apoptosis. It was also found to induce ATM and H2AX phosphorylation in cancer cells. Inhibition of ATM helps to control cell proliferation leading to cell cycle arrest [42]. Another study examined the effect of kaempferol on HT-29 human colon cancer cells. Cells were treated with 0−60 μmol/L concentration of kaempferol. MTT assay was used to evaluate its effect on cell proliferation and [(3)H]thymidine incorporation assay determined its effect on DNA synthesis. Cell cycle phase distribution was also studied using fluorescence-activated cell sorting analyses. The activity of cyclin-dependent kinase (CDK)s was measured using in vitro kinase assays and western blot analyses revealed the expression of proteins in various stages of the cell cycle. It was found that the kaempferol reduced the number of viable cells and brought the dose-dependent incorporation of [(3)H]thymidine in HT29 cell DNA. Cell cycle was arrested at the G1 stage within 6 h, and G2/M arrest required 12 h. The protein expression of CDK2, CDK4, cyclins D1, cyclin E, and cyclin A and the activity of CDK2 and CDK4 was inhibited. The phosphorylation retinoblastoma protein was also suppressed. Further, it reduced the concentration of Cdc25C, Cdc2, and cyclin B1 proteins. Cdc2 activity was similarly decreased. The study found that kaempferol suppresses CDK2, CDK4, and Cdc2 activity, causing cell cycle arrest at the G1 and G2/M stages. Kaempferol's ability to suppress colon cancer growth may be linked to its ability to induce cell cycle arrest in colon cancer cells [43, 44].
Another study conducted to study the activity of curcumin and kaempferol on the DLD-1 colon cancer cell line of epithelial origin indicated the inhibitory role of both phytochemicals during cell proliferation [45]. Significant antiproliferative activity of kaempferol was observed at high levels, while curcumin was effective in relatively low concentrations. Synergistic antiproliferative effects were evident. Kaempferol in low concentration was very effective when combined with curcumin at 12.5 μM (IC50). This combination of kaempferol and curcumin showed significant antiproliferative activity against colon cancer cells (HCT-15) and human normal lymphocytes [46, 47]. Recent studies in the literature clarified that kaempferol is a promising phytopharmaceutical used in the management of colon cancer via mediating inflammation and signal transduction pathways [48].
4 ISORHAMNETIN
Isorhamnetin is widely distributed in fruits (i.e., Hippophae rhamnoides L). Isorhamnetin, a 3′-O-methylated metabolite of quercetin, has lethal effects on human colon cancer cells via affecting cell cycle, cell death, and proliferation in human colon carcinoma (HCT-116) cells. The number of cells was discovered to rise during the G2/M phase. With an IC50 of 72 M, the MTT assay demonstrated its effectiveness in a dose- and time-dependent manner. It initiates apoptosis and necrosis as suggested by flow cytometry and fluorescence microscopy. Low serum levels initiate cell cycle progression to the G0/G1 phase and positively influence cell death leading to cell necrosis or apoptotic processes. Here, the G2/M stage may be a target to inhibit HCT-116 cell growth. The above activities suggest the potential chemopreventive property of isorhamnetin [49]. Another report suggests oncogenic Src and β-catenin as potential mediators for their chemopreventive action in colorectal cancer. Advanced adenoma relapse could be prevented using an isorhamnetin-rich diet in a poly-prevention trial. Dietary isorhamnetin was found to be effective in controlling the tumor burden, and tumor number by 59% and 35%. The mortality rates were also decreased to 62% in a study involving colorectal tumorigenesis of FVB/N mice. The study used azoxymethane-treated cells, which were further treated with dextran sodium sulfate (DSS). DSS is a colonic irritant and azoxymethane is a known chemical carcinogen. It was observed using immune histochemical analysis and histopathology studies that dietary isorhamnetin controlled DSS-induced inflammation faster than the control diet. This response was mediated by stimulation of C-terminal Src kinase (CSK) and inhibition of β-catenin nuclear translocation. c-Src activation, which was induced by DSS was also suppressed. CSK is a negative regulator of of Src family of tyrosine kinases [50]. A similar mechanism was observed in another study using HT-29 colon cancer cells, where RNA interference helped to stimulate CSK expression. As evident in studies conducted over human CRC such as HT-29, HCT116, and SW480; it may be concluded that dietary isorhamnetin mediates its activity by inhibiting PI3K-Akt-mTOR pathway and anti-inflammatory mechanisms and positively modulating the expression of CSK and negatively influencing the oncogenic Src activity and expression of cyclin B1 protein. It also modulated the phosphorylation levels of phosph-p70S6 kinase, phosph-4E-BP1 (t37/46), and protein Akt (ser473) [51]. Isorhamnetin is identified to encourage vital anticancer activity via numerous signaling pathways (PI3K/AKT, and NF-κB) and has shown promising activity against cancer [52, 53]. Recent data itself supports the fact that isorhamnetin has anti-colon rectal cancer activity. This has proven that this may be due to upregulating factors responsible for cell cycle arrest or cell death.
5 MYRECITIN
Myricetin (3,3′,4′,5,5,7′-hexahydroxyflavone) is widely distributed in berries, tea, and red wine. It exhibits structural similarity with quercetin. Studies conducted over HT-29 cells, Caco-2, SW480, COLO 205, VACO-235, and T84, show its inhibitory effect on cell growth, induction of apoptosis, inhibition of cell multiplication, and antimetastatic properties. It is believed to act via inhibitory effects over EGFR kinases, matrix metalloproteinase isoform 2 activity modulation of apopain activity, and auto-oxidation process [54, 55]. Its effects on rat colon carcinogenesis were investigated using 1,2 dimethyl hydrazine as an inducer. Overall it reduced the number of tumors and the progress of the disease. Liver TBARS were significantly reduced. The bacterial enzyme activity was significantly reduced at a dose of 50 and 100 mg/kg. Glutathione peroxidase, GSH, and catalase activity were all considerably increased in a dose-dependent manner [56]. In HCT-15 cells, myricetin was discovered to cause apoptosis. To increase the BCL2-associated X protein/B-cell lymphoma 2 ratio, it likely functions by releasing the positive apoptosis influencer from the mitochondrial membrane. However, the cleavage of caspase-3 and caspase-9 was unaffected [57]. Additional in vivo research may shed light on specific pathways [58-61]. Myricetin's anticancer activity and ability to operate on S4-10 have been demonstrated in vitro and in vivo during experiments involving the proliferation of A549 cells [62, 63].
6 CONCLUSION AND RELEVANCE TO FUTURE RESEARCH
Flavonols are new hope, a gift by nature, not only because this class assists in normal cell growth by apoptosis, metastasis, and antiproliferative activity using various molecular cascade of events, but it seems to elevate general well-being silently because they are consumed widely without prescription as a dietary ingredient. However, a cautious approach is recommended as these studies have not been proven through clinical trials. These studies have used a concentration range which is difficult to achieve through dietary sources, as the free form of many flavonols shows low aqueous solubility and high molecular weight leading to low bioavailability, extensive metabolism, and rapid elimination. Poor vascular/oral bioavailability and bitter taste further create a challenge for formulators. The clinical relevance of conjugated forms of their metabolites is required to be investigated as they may not be as effective as compared to their precursors. The stability of these compounds is another concern. Flavonols are sensitive to various environmental conditions such as light and heat and rapidly undergo oxidation and hydrolysis. The exposure time is still to be manipulated and understood before utilizing their potential as colorectal cancer preventive agents. The effect of dose with reference to the treatment period is required to be established. The question remains whether their dietary concentration will help or only therapeutic formulation will be effective. The synergistic effects of these compounds are required to be further explored. Whether its bioavailability will improve after targeted intestinal absorption and metabolism, is still to be answered. Their safety and toxicity-related concerns are required to be addressed as this class utilizes various critical biochemical and molecular cascades of events to express their therapeutic efficacy. The modulation of these pathways may interfere with the metabolism of other compounds, hampering other required events, and antagonizing systemic drug effects. Pro-drug design (i.e., methoxylated flavonoids) may also be helpful to achieve some goals. Methoxylated flavonoids are promising chemopreventive compounds, which act as prodrugs in events associated with CYP1A1 and CYP1 metabolism. Sufficient in vitro studies have reported their chemopreventive properties. If, flavonols and their prodrugs could be further explored in vivo, a new arena for research may emerge.
AUTHOR CONTRIBUTIONS
Nitin Dubey: Investigation (equal), methodology (equal), writing—original draft (equal), writing—review and editing (equal). Nidhi Dubey: Conceptualization (equal), supervision (equal), writing—original draft (equal), writing—review and editing (equal). Upendra Bhadoria: Methodology (equal), writing—original draft (equal), writing—review and editing (equal). Kamal Shah: Investigation (equal), methodology (equal), writing—original draft (equal), writing—review and editing (equal). Nagendra Singh Chauhan: Investigation (equal), supervision (equal), writing—review and editing (equal).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.




