Individualized elective irradiation of the clinically node-negative neck in definitive radiotherapy for head and neck squamous cell carcinoma
Abstract
Background
Oral cavity (OC), oropharyngeal (OP), hypopharyngeal (HP), and laryngeal (LA) squamous cell carcinoma (SCC) have a high incidence of regional lymph node metastasis (LNM). Elective irradiation for clinically node-negative neck is routinely administered to treat lymph nodes harboring occult metastasis. However, the optimal elective irradiation schemes are still inconclusive. In this study, we aimed to establish individualized elective irradiation schemes for the ipsilateral and contralateral node-negative neck of these four types of cancer.
Methods
From July 2005 to December 2018, 793 patients with OC-SCC, 464 with OP-SCC, 413 with HP-SCC, and 645 with LA-SCC were recruited retrospectively. Based on the actual incidence of LNM and the tumor characteristics, risk factors for contralateral LNM, as well as node level coverage schemes for elective irradiation, were determined using logistic regression analysis. Additionally, we developed a publicly available online tool to facilitate the widespread clinical use of these schemes.
Results
For the ipsilateral node-negative neck, elective irradiation at levels I-III for OC-SCC and levels II-IVa for OP-, HP- and LA-SCC are generally recommended. In addition, level VIIa should be included in patients with OP-SCC. Multivariate analyses revealed that posterior hypopharyngeal wall and post-cricoid region involvement were independently associated with level VIIa metastasis in HP-SCC (all P < 0.05). For the contralateral node-negative neck, multivariate analyses revealed that ipsilateral N2b2-N3, tumors with body midline involvement, and degree of tumor invasion were the independent factors for contralateral LNM (all P < 0.05). In patients who require contralateral neck irradiation, levels I-II are recommended for OC-SCC, and additional level III is recommended for patients with ipsilateral N3 disease. Levels II-III are recommended for OP-, HP-, and LA-SCC, and additional level IVa is recommended for patients with advanced T or ipsilateral N classifications. Furthermore, additional level VIIa is recommended only for OP-SCC with T4 and ipsilateral N3 disease.
Conclusion
Based on our findings, we suggest that individualized and computer-aided elective irradiation schemes could reduce irradiation volumes in OC-, OP- and HP-SCC patients, as compared to current guidelines, and could thus positively impact the patients' quality of life after radiotherapy.
Abbreviations
-
- CI
-
- confidence interval
-
- Contra
-
- contralateral neck
-
- HNSCC
-
- Head and neck squamous cell carcinoma
-
- HP
-
- Hypopharyngeal
-
- ICRU
-
- International Commission on Radiation Units and Measurements
-
- Ipsi
-
- ipsilateral neck
-
- LA
-
- Laryngeal
-
- LN
-
- Lymph node
-
- LNM
-
- Lymph node metastasis
-
- MID
-
- minimal axial diameter
-
- OC
-
- Oral cavity
-
- OP
-
- Oropharyngeal
-
- OR
-
- odds ratio
-
- PACS
-
- Picture Archiving and Communication System
-
- SCC
-
- Squamous cell carcinoma
-
- UICC/AJCC
-
- American Joint Committee on Cancer and Union for International Cancer Control
1 INTRODUCTION
Oral cavity (OC)-squamous cell carcinoma (SCC), oropharyngeal (OP)-SCC, hypopharyngeal (HP)-SCC, and laryngeal (LA)-SCC have the highest incidences of all types of head and neck squamous cell carcinoma (HNSCC) [1]. Due to the vast submucosal lymphatic capillary network, these four cancers have a high propensity to metastasize to regional lymph nodes (LNs). Historical data have shown that 36%–70% of patients have clinically detectable regional lymph node metastasis (LNM). Even in the patients with negative LNs diagnosed by clinical examination, namely patients with clinically node-negative neck, there was still a 33% probability of pathologically confirmed regional LNM [2, 3]. These microscopically-identified occult metastatic LNs could develop into adenopathy during follow-up if untreated. Accordingly, the effectiveness of elective irradiation in the treatment of occult metastatic LNs was first demonstrated by Fletcher and supported later on by others [4-6]. Since then, elective irradiation of the clinically node-negative neck is routinely administered to treat LNs harboring occult metastasis.
In the process of elective irradiation, the selection of appropriate irradiation volume is one of the most important issue that needs to be addressed because insufficient radiation volume could impair tumor control and excessive radiation volume could cause unnecessary damage to normal tissue of the neck [7]. At present, for the selection of the elective irradiation volume for HNSCC, the most widely adopted guidelines were proposed by Grégoire et al. in 2000 [2], with reference to historically pathological data, and were recently updated in 2018 and 2019 [8, 9]. Nonetheless, these guidelines still have several limitations worth optimizing. First, tumor size, tumor grade, and tumor site are important risk factors for lymph node metastasis that should be taken into consideration for treatment decisions [10-12], while the guidelines ignore the influence of these various tumor characteristics on LNM in the ipsilateral and contralateral neck. Second, the guidelines recommend unified irradiation schemes for the bilateral node-negative neck, although studies have constantly confirmed that the LNM rate of the contralateral neck is significantly lower than that of the ipsilateral neck [13, 14]. Taken together, to achieve more individualized precision radiotherapy of patients with HNSCC, the contralateral neck should be distinguished from the ipsilateral neck, and the elective irradiation guidelines should be refined based on corresponding tumor characteristics.
Therefore, the aim of the present study was to establish individualized elective irradiation schemes for the ipsilateral and contralateral node-negative neck, respectively, based on the actual LNM rate and corresponding tumor characteristics in a large patient cohort. Further, we developed an online tool to facilitate the widespread clinical use of the proposed elective irradiation schemes.
2 MATERIALS AND METHODS
2.1 Patients
The data of consecutive patients with non-metastatic, newly diagnosed pathologically-proven HNSCC in Sun Yat-Sen University Cancer Center (Guangzhou, China) between July 2005 and December 2018, were retrospectively retrieved for this study. We included patients who underwent baseline contrast-enhanced computed tomography or magnetic resonance imaging scans of the head and neck region, and all the images were acquired from our institutional Picture Archiving and Communication System (PACS, General Electric Healthcare). We excluded patients who had a history of head and neck radiotherapy or surgery before they were diagnosed with OP-SCC/OC-SCC/HP-SCC/LA-SCC at our institution and those with an insufficient scanning scope for covering the primary site and neck. All patients were restaged according to the 7th edition of the American Joint Committee on Cancer and Union for International Cancer Control (UICC/AJCC) staging system [15]. For primary tumors, the pathological staging system was used for patients with surgery as the initial treatment after diagnosis; otherwise, the clinical staging system was used. For cervical LNs, the clinical staging system was used for all patients. The study was approved by the institutional review board (IRB reference No.: B2020-025-01) and the requirement to obtain informed consent was waived.
2.2 Diagnosis of clinically metastatic lymph nodes
In this study, clinically metastatic LNs were diagnosed from the results of pretreatment imaging examinations. The imaging diagnostic criteria for clinically metastatic LNs were: (1) the minimal axial diameter (MID) of the cervical LN was ≥ 10 mm, and the MID of the retropharyngeal LN was ≥ 5 mm; (2) nodal grouping: three or more contiguous LNs and any one of them had a MID ≥ 8 mm, and; (3) presence of signs of necrosis or extra-capsular invasion in any sized LN.
2.3 Primary tumor and lymph node characteristics
For primary tumors, four characteristics were determined and recorded, according to physical examination, endoscopy, and imaging examinations. The first characteristic was the tumor subsite. The oral cavity includes seven subsites (buccal mucosa, retromolar gingiva, alveolar ridge, hard palate, oral tongue, floor of the mouth, and mucosal lip); the oropharynx was divided into four subsites (tonsil, soft palate, base of the tongue, and oropharyngeal wall); and the hypopharynx and larynx both include three subsites (hypopharynx: pyriform sinuses, post-cricoid region, and hypopharyngeal wall; and larynx: supraglottis, subglottis, and glottis). The second characteristic was the presence of a unilateral or bilateral tumor. Tumors that deviated to the left or right side were defined as unilateral tumors, otherwise, they were defined as bilateral tumors. The third characteristic was body midline involvement (presence/absence). The fourth characteristic was the involvement of certain structures (oral cavity involvement in OP-SCC; oropharynx, post-cricoid, posterior hypopharyngeal wall, and esophagus involvement in HP-SCC; and post-cricoid, posterior hypopharyngeal wall, and subglottis involvement in LA-SCC). In regard to LNs, if the primary tumor was defined as a bilateral tumor, the LNs on both sides of the neck were also defined as ipsilateral LNs; otherwise, the LNs were separated into ipsilateral or contralateral LNs. It is noteworthy that the levels Ia, VIa, and VIb are located in the midline region of the body and bilateral sides of these levels are connected. Therefore, these levels were consistently classified as the ipsilateral levels and the LNs located in these levels were classified as the ipsilateral LNs in data analysis. In addition, to detailly explore the influence of ipsilateral LN metastasis on contralateral LN metastasis, we separately defined the ipsilateral N classification according to the features of metastatic LNs in the ipsilateral neck with reference to the 7th edition of the UICC/AJCC staging system. Specifically, we further divided N2b into N2b1 (distribution of multiple LNs in one level of the ipsilateral neck) and N2b2 (distribution of multiple LNs in more than one level of the ipsilateral neck).
2.4 Elective irradiation of the ipsilateral node-negative neck
We aimed to propose elective irradiation schemes for the ipsilateral node-negative neck in OP-, OC-, HP-, and LA-SCC. The LNM rate of a certain level in the ipsilateral neck was defined as the ratio of the number of patients with clinically metastatic LNs on this level and the total number of patients with a certain type of cancer. First, we calculated the LNM rates for levels II-V of these four types of cancer. Given that level I is the first station of lymphatic drainage in OC-SCC and level VIIa (retropharyngeal LNs) is the first station in OP-SCC [16], the LNM rates in level I for OC-SCC and level VIIa for OP-SCC were also calculated. Although there is no consensus on what probability of LNs metastasis is considered relevant for therapy, the International Commission on Radiation Units and Measurements (ICRU) Report 83 [17] proposed that typically a probability of LNs metastasis higher than from 5% to 10% should be assumed to require treatment. In previous literature, Eisbruch et al. [18] also proposed guidelines of elective irradiation for HNSCC based on a 5% to 10% probability of LNs metastasis. Therefore, in this study, we supposed that levels with metastasis rates ≥5% were considered necessary to undergo elective irradiation. The levels with metastasis rates <5% were further analyzed in patient subgroups with different T classifications.
Next, for certain levels (level Ib for OP-SCC, level VI and VIIa for HP-SCC, and level VI for LA-SCC), the LNM rates are generally very low. However, previous literature [19-22] reported that tumor size, tumor invasion structure, and other clinical factors were significantly associated with LNM in these levels; and the LNM in these levels were associated with poor survival of the patients. Therefore, the univariate and multivariate binary logistic regression analyses were performed to define high-risk factors for LNM in these levels. Covariates included histological grade, T classification, and structures with tumor involvement. We recommended elective irradiation of these levels in patients with any high-risk factors.
2.5 Elective irradiation of the contralateral node-negative neck
In patients with unilateral tumors, high-risk factors for contralateral LNM were defined using univariate and multivariate binary logistic regression analyses. The covariates included histological grade, T classification, tumor subsite, body midline involvement (presence/absence), structures with tumor involvement, and ipsilateral N classification. Elective irradiation of the contralateral neck was recommended in patients with any high-risk factors, and the LNM rate of a certain level in the contralateral neck was defined as a ratio of the number of patients with clinically metastatic LNs on this level and the number of patients who were defined as requiring contralateral neck elective irradiation. Next, based on these patients, we also proposed elective irradiation schemes for the contralateral node-negative neck by applying the same methods used to analyze the ipsilateral neck. However, the levels with metastasis rates <5% were further calculated in patient subgroups with different T classifications and ipsilateral N classifications.
2.6 Construction of an online tool for the widespread clinical use of our proposed elective irradiation schemes
To facilitate the widespread clinical use of our proposals, we constructed a publicly available online tool. The tool was developed based on the React front-end framework and KOA back-end framework. PostGreSQL was used as the server database to store data information. To improve the development efficiency and stability, several third-party plugins such as dicomParser, cornerstoneTools and WADOImageLoader, were imported.
The web server of this tool consists of application layer, analysis layer, and data layer. The application layer was used for user interaction, Dicom viewer, and exhibition of analysis results. The analysis layer uses Prolog, natural language analysis, to develop the deductive reasoning process and store reference data. The selection criteria entered by the users are analyzed and the results are finally returned. The data layer is mainly used to store image data and analysis results. The system architecture diagram of this on-line tool is shown in Figure S1.
2.7 Statistical analysis
All variables used in our study were categorical variables. Univariate and multivariate binary logistic regression analyses were performed to define risk factors for LNs metastasis in certain nodal levels and contralateral neck. The odds ratio (OR) and its 95% confidence interval (CI) were calculated from the logistic regression analysis. Univariate and multivariate binary logistic regression analyses were performed by using Statistical Product and Service Solutions (IBM SPSS, version 22.0; Armonk, NY, USA). Statistical significance was set at a two-tailed P < 0.05.
3 RESULTS
3.1 Patients distributions
A total of 2315 patients with HNSCC were included in this study, comprising 793 patients with OC-SCC, 464 with OP-SCC, 413 with HP-SCC, and 645 with LA-SCC. The clinicopathological characteristics of the enrolled patients are shown in Table 1.
| Number of patients (%) | ||||
|---|---|---|---|---|
| Characteristics | OC-SCC n = 793 | OP-SCC n = 464 | HP-SCC n = 413 | LA-SCC n = 645 |
| Sex | ||||
| Male | 569 (71.8) | 409 (88.1) | 393 (95.2) | 624 (96.7) |
| Female | 224 (28.2) | 55 (11.9) | 20 (4.8) | 21 (3.3) |
| Age | ||||
| Median (range, yr.) | 60 (12-97) | 60 (22-89) | 63 (28-93) | 65 (32-92) |
| >60 | 390 (49.2) | 218 (47) | 254 (61.5) | 446 (69.1) |
| ≤60 | 403 (50.8) | 246 (53) | 159 (38.5) | 199 (30.9) |
| Histologic grade* | ||||
| GX | 33 (4.2) | 33 (7.1) | 39 (9.4) | 51 (7.9) |
| G1-G2 | 724 (91.3) | 331 (71.3) | 285 (69.0) | 501 (77.7) |
| G3-G4 | 36 (4.5) | 100 (21.6) | 89 (21.5) | 93 (14.4) |
| T classification | ||||
| T1 | 216 (27.2) | 78 (16.8) | 17 (4.1) | 193 (29.9) |
| T2 | 312 (39.3) | 165 (35.6) | 142 (34.4) | 165 (25.6) |
| T3 | 160 (20.2) | 80 (17.2) | 117 (28.3) | 195 (30.2) |
| T4 | 105 (13.2) | 141 (30.4) | 137 (33.2) | 92 (14.3) |
| N classification | ||||
| N0 | 485 (61.2) | 139 (30.0) | 100 (24.2) | 477 (74.0) |
| N1 | 116 (14.6) | 75 (16.2) | 93 (22.5) | 57 (8.8) |
| N2a | 3 (0.4) | 19 (4.1) | 5 (1.2) | 2 (0.3) |
| N2b | 143 (18.0) | 137 (29.5) | 114 (27.6) | 44 (6.8) |
| N2c | 28 (3.5) | 54 (11.6) | 44 (10.7) | 46 (7.1) |
| N3 | 18 (2.3) | 40 (8.6) | 57 (13.8) | 19 (2.9) |
| Stage | ||||
| I | 191 (24.1) | 37 (8.0) | 6 (1.5) | 187 (29.0) |
| II | 199 (25.1) | 52 (11.2) | 38 (9.2) | 134 (20.8) |
| III | 161 (20.3) | 75 (16.2) | 100 (24.2) | 157 (24.3) |
| IV | 242 (30.5) | 300 (64.7) | 269 (65.1) | 167 (25.9) |
| Ipsilateral N classification† | ||||
| N0 | 487 (61.4) | 140 (30.2) | 104 (25.2) | 480 (74.4) |
| N1 | 120 (15.1) | 86 (18.5) | 98 (23.7) | 62 (9.6) |
| N2a | 4 (0.5) | 20 (4.3) | 7 (1.7) | 4 (0.6) |
| N2b1 | 38 (4.8) | 45 (9.7) | 25 (6.1) | 20 (3.1) |
| N2b2 | 126 (15.9) | 133 (28.7) | 122 (29.5) | 59 (9.1) |
| N3 | 18 (2.3) | 40 (8.6) | 57 (13.8) | 20 (3.1) |
| Primary tumor laterality | ||||
| Bilateral | 22 (2.8) | 32 (6.9) | 57 (13.8) | 129 (20.0) |
| Unilateral | 771 (97.2) | 432 (93.1) | 356 (86.2) | 516 (80.0) |
| Body midline involvement | ||||
| Present | 160 (20.2) | 151 (32.5) | 224 (54.2) | 346 (53.6) |
| Absent | 633 (79.8) | 313 (67.5) | 189 (45.8) | 299 (46.4) |
- Data are number of patients, with the percentage in parentheses;
- * GX, grade could not be assessed, G1, well-differentiated, G2, moderately differentiated, G3, poorly differentiated, G4, undifferentiated;
- † To better characterize LNs metastasis of the ipsilateral neck in patients with unilateral tumors, we separately defined the ipsilateral N classification according to the features of metastatic LNs in the ipsilateral neck with reference to the 7th edition of the UICC/AJCC staging system. Specifically, we continue to divide N2b into N2b1 (multiple LNs are distributed in one level of the ipsilateral neck) and N2b2 (multiple LNs are distributed in more than one level of the ipsilateral neck);
- Abbreviations: HP, hypopharyngeal; LA, laryngeal; OC, oral cavity; OP, oropharyngeal; SCC, squamous cell carcinoma.
3.2 Distribution and incidence of clinically metastatic lymph nodes
The distribution and incidence of clinically metastatic LNs in patients with OC-SCC, OP-SCC, HP-SCC, and LA-SCC are shown in Table 2 and Figure 1. Table S1 and S2 present these results by various tumor subsites. According to the results, 1114 (48.1%) out of 2315 patients presented with LNM. Patients with HP-SCC had the highest incidence of LNM (313/413, 75.8%), followed by patients with OP-SCC (325/464, 70.0%), OC-SCC (308/793, 38.8%), and LA-SCC (168/645, 26.0%). The incidences of contralateral LNM, as ranked from high to low, were as follows: HP-SCC (59/413, 14.3%), OP-SCC (60/464, 12.9%), LA-SCC (49/645, 7.6%), and OC-SCC (32/793, 4.0%).
| OC-SCC (n = 793) | OP-SCC (n = 464) | HP-SCC (n = 413) | LA-SCC (n = 645) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Levels | Ipsi (%) | Contra (%) | Total (%) | Ipsi (%) | Contra (%) | Total (%) | Ipsi (%) | Contra (%) | Total (%) | Ipsi (%) | Contra (%) | Total (%) |
| Ia* | 29 (3.7) | NA | 29 (3.7) | 2 (0.4) | NA | 2 (0.4) | 2 (0.5) | NA | 2 (0.5) | 0 (0) | NA | 0 (0) |
| Ib | 153 (19.3) | 14 (1.8) | 160 (20.2) | 14 (3.0) | 0 (0) | 14 (3.0) | 3 (0.7) | 3 (0.7) | 5 (1.2) | 3 (0.5) | 1 (0.2) | 3 (0.5) |
| II | 208 (26.2) | 13 (1.6) | 209 (26.4) | 305 (65.7) | 48 (10.3) | 308 (66.4) | 256 (62.0) | 37 (9.0) | 259 (62.7) | 128 (19.8) | 40 (6.2) | 134 (20.8) |
| III | 82 (10.3) | 4 (0.5) | 85 (10.7) | 132 (28.4) | 14 (3.0) | 137 (29.5) | 170 (41.2) | 19 (4.6) | 175 (42.4) | 86 (13.3) | 16 (2.5) | 93 (14.4) |
| IVa | 15 (1.9) | 1 (0.1) | 16 (2.0) | 28 (6.0) | 4 (0.9) | 31 (6.7) | 64 (15.5) | 10 (2.4) | 69 (16.7) | 34 (5.3) | 7 (1.1) | 39 (6.0) |
| IVb | 0 (0) | 0 (0) | 0 (0) | 4 (0.9) | 0 (0) | 4 (0.9) | 4 (1.0) | 3 (0.7) | 3 (0.7) | 1 (0.2) | 0 (0) | 1 (0.2) |
| Va | 9 (1.1) | 0 (0) | 9 (1.1) | 15 (3.2) | 3 (0.6) | 18 (3.9) | 22 (5.3) | 4 (1.0) | 24 (5.8) | 7 (1.1) | 2 (0.3) | 8 (1.2) |
| Vb | 0 (0) | 0 (0) | 0 (0) | 8 (1.7) | 0 (0) | 8 (1.7) | 5 (1.2) | 1 (0.2) | 5 (1.2) | 1 (0.2) | 1 (0.2) | 2 (0.3) |
| Vc | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| VIa* | 3 (0.4) | NA | 3 (0.4) | 0 (0) | NA | 0 (0) | 0 (0) | NA | 0 (0) | 7 (1.1) | NA | 7 (1.1) |
| VIb* | 0 (0) | NA | 0 (0) | 0 (0) | NA | 0 (0) | 27 (6.5) | NA | 27 (6.5) | 7 (1.1) | NA | 7 (1.1) |
| VIIa | 14 (1.8) | 4 (0.5) | 16 (2.0) | 81 (17.5) | 10 (2.2) | 84 (18.1) | 38 (9.2) | 9 (2.2) | 44 (10.7) | 5 (0.8) | 0 (0) | 5 (0.8) |
| VIIb | 2 (0.3) | 0 (0) | 2 (0.3) | 2 (0.4) | 1 (0.2) | 3 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VIII | 1 (0.1) | 0 (0) | 1 (0.1) | 3 (0.6) | 1 (0.2) | 4 (0.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IXa | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Xa | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Xb | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 306 (38.6) | 32 (4.0) | 308 (38.8) | 324 (69.8) | 60 (12.9) | 325 (70.0) | 309 (74.8) | 59 (14.3) | 313 (75.8) | 165 (25.6) | 49 (7.6) | 168 (26.0) |
- Data are number of patients, with the percentage in parentheses; NA, figures are not available;
- * Levels Ia, VIa, and VIb are located in the midline region of the body and were classified as the ipsilateral levels in data analysis, so there is no data of contralateral lymph node metastasis in these levels;
- Abbreviations: HP, hypopharyngeal; LA, laryngeal; OC, oral cavity; OP, oropharyngeal; SCC, squamous cell carcinoma; Ipsi, ipsilateral neck; Contra, contralateral neck.
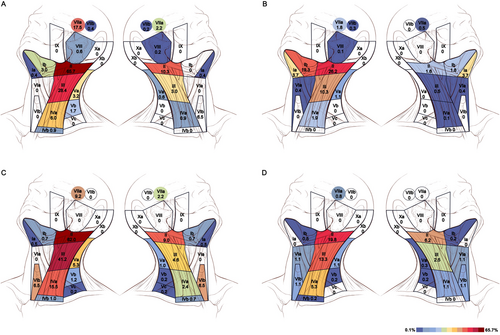
Distribution and incidence of clinically metastatic lymph nodes in patients with OP-SCC (A), OC-SCC (B), HP-SCC (C), and LA-SCC (D). The left and right neck were substituted for the ipsilateral and contralateral neck to illustrate the pattern of lymph node metastasis. The numbers represent the incidence of lymph node metastasis in each nodal level of the ipsilateral and contralateral neck. Color wash changing from blue through yellow to red indicates incidence increase. Levels Ia, VIa, and VIb are located in the midline region of the body. OP, oropharyngeal; OC, oral cavity; HP, hypopharyngeal; LA, laryngeal; SCC, squamous cell carcinoma
3.3 Elective irradiation of the ipsilateral node-negative neck
Herein, OP-SCC was used as an example to demonstrate the results, while the results of the other three cancers are detailed in the Supplementary Materials.
The study flow diagram of OP-SCC is shown in Figure 2. Of the 464 patients with OP-SCC, the LNM rates of the ipsilateral neck were 17.5% (n = 81) for level VIIa, 65.7% (n = 305) for level II, 28.4% (n = 132) for level III, 6.0% (n = 28) for level IVa, 3.2% (n = 15) for level Va, 1.7% (n = 8) for level Vb, 0.9% (n = 4) for level IVb and 0% for level Vc (Table 2). According to the predefined 5% threshold, we recommend elective irradiation of levels VIIa and II-IVa for the ipsilateral node-negative necks. When analyzed in patient subgroups with different T classifications, the LNM rates of levels IVb, Va, Vb, and Vc were lower than 5%, even in patients with advanced primary disease (Table S3); therefore, elective irradiation of levels IVb and Va-Vc is not suggested for ipsilateral node-negative necks.
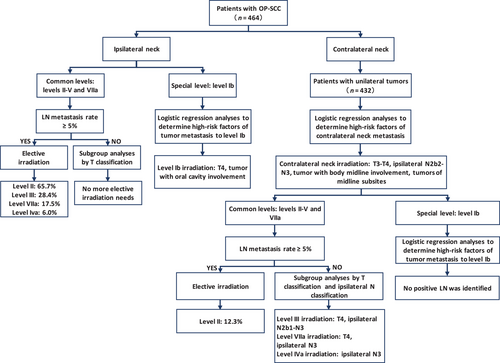
Study flow diagram of OP-SCC. OP, oropharyngeal; SCC, squamous cell carcinoma; LN, lymph node
Among the 464 patients with OP-SCC, 3.0% (n = 14) had ipsilateral level Ib metastasis. Univariate and multivariate logistic regression analyses demonstrated that T4 and oral cavity involvement were independently associated with ipsilateral level Ib metastasis (T4 vs. T1-3, 7.8% vs. 0.9%; OR 6.3; 95% CI 1.7–24.0; P = 0.007; oral cavity involvement, presence vs. absence, 8.0% vs. 1.4%; OR 3.7; 95% CI 1.2–11.7; P = 0.028; Table S4 and S5). Therefore, we suggest elective irradiation of the ipsilateral level Ib in OP-SCC patients with T4 disease or oral cavity involvement.
3.4 Elective irradiation of the contralateral node-negative neck
Among OP-SCC patients with unilateral tumors, 13.9% (60/432) had contralateral LNM. Univariate and multivariate analyses demonstrated that T3, T4, body midline involvement, tumors of midline subsites, and ipsilateral N2b2-N3 were independently associated with higher incidences of contralateral LNM (T3-4 vs. T1-2, 22.1% vs. 6.6%; OR, 2.5; 95% CI, 1.2–5.2; P = 0.012; body midline involvement, presence vs. absence, 29.8% vs. 7.7%; OR, 2.8; 95% CI, 1.4–5.3; P = 0.002; tumors of midline subsites, presence vs. absence, 18.0% vs. 7.2%; OR, 2.8; 95% CI, 1.5–5.3; P = 0.012; N2b2-N3 vs. N0-N2b1, 26.3% vs. 6.0%; OR, 4.9; 95% CI, 2.6-9.4; P < 0.001; Table S6 and Table 3). Therefore, contralateral neck irradiation is recommended for patients with any of the high-risk factors. Accordingly, in our cohort, 86.3% (373/432) of OP-SCC patients with unilateral tumors were defined as requiring contralateral neck irradiation. The contralateral LNM rate was significantly higher in patients requiring contralateral neck irradiation than in those not requiring irradiation (58/373, 15.5% vs. 2/59, 3.4%, P = 0.024).
| Tumor variables | Incidence of contralateral LNs metastasis (%) | OR | 95% CI | P |
|---|---|---|---|---|
| T classification | 2.5 | 1.2-5.2 | 0.012 | |
| T1-2 | 15/228 (6.6) | |||
| T3-4 | 45/204 (22.1) | |||
| Ipsilateral N classification* | 4.9 | 2.6-9.4 | < 0.001 | |
| N0-N2b1 | 16/265 (6.0) | |||
| N2b2-N3 | 44/167 (26.3) | |||
| Body midline involvement | 2.8 | 1.4-5.3 | 0.002 | |
| Absent | 24/311 (7.7) | |||
| Present | 36/121 (29.8) | |||
| Primary tumor subsites† | 2.8 | 1.5-5.3 | 0.012 | |
| Non-midline structure | 12/166 (7.2) | |||
| Midline structure | 48/266 (18.0) |
- Data are number of patients, with the percentage in parentheses;
- * To better characterize LNs metastasis of the ipsilateral neck in patients with unilateral tumors, we separately defined the ipsilateral N classification according to the features of metastatic LNs in the ipsilateral neck with reference to the 7th edition of the UICC/AJCC staging system. Specifically, N2b1 denotes that multiple LNs are distributed in one level of the ipsilateral neck; N2b2 denotes that multiple LNs are distributed in more than one levels of the ipsilateral neck;
- † Primary tumor subsites: non-midline structure (tonsil), midline structure (soft palate, base of the tongue and pharyngeal wall);
- Abbreviations: LNs, lymph nodes; OP, oropharyngeal; SCC, squamous cell carcinoma.
In 373 patients with OP-SCC requiring contralateral neck irradiation, the LNM rates of the contralateral neck were 2.7% (n = 10) for level VIIa, 12.3% (n = 46) for level II, 3.8% (n = 14) for level III, 1.1% (n = 4) for level IVa, 0.8% (n = 3) for level Va, and 0% for levels IVb, Vb, and Vc (Table S7). Therefore, we recommend elective irradiation of contralateral level II for all these patients. Subgroup analysis using patients with different T classifications and ipsilateral N classifications showed that the LNM rates of contralateral level VIIa in patients with T4 disease (9/128, 7%) and ipsilateral N3 (2/37, 5.4%) disease; of contralateral level III in patients with T4 (10/128, 7.8%), ipsilateral N2b1 (2/33, 6.1%), N2b2 (9/130, 6.9%) and N3 (3/37, 8.1%) disease; and of contralateral level IVa in patients with ipsilateral N3 disease (2/37, 5.4%) exceeded 5% (Table S7). Therefore, elective irradiation of contralateral level VIIa is recommended in patients with T4 disease and ipsilateral N3 disease; of contralateral level III in patients with T4 disease and ipsilateral N2b1-N3 disease; and of contralateral level IVa in patients with ipsilateral N3 disease. Given that no clinically metastatic LNs were identified in contralateral level Ib among all patients, elective irradiation of contralateral level Ib is not recommended for patients with a contralateral node-negative neck.
3.5 Summary of elective irradiation schemes for OP-SCC
For the ipsilateral node-negative neck, elective irradiation of levels VIIa and II-IVa is recommended for all patients. Additionally, ipsilateral level Ib is suggested in patients with T4 disease or oral cavity involvement. For the contralateral node-negative neck, elective irradiation is proposed for patients with T3-T4 disease, ipsilateral N2b2-N3 disease, tumors with body midline involvement, and tumors of midline subsites (including the base of the tongue, soft palate, and oropharyngeal wall). In all these patients, elective irradiation of contralateral level II is recommended. Additionally, contralateral level III irradiation is recommended for patients with T4 and ipsilateral N2b1-N3 disease; contralateral level VIIa irradiation is recommended for patients with T4 and ipsilateral N3 disease, and; contralateral level IVa irradiation is recommended for patients with ipsilateral N3 disease. Our proposals for the elective irradiation schemes for OP-SCC, as well as OC-SCC, HP-SCC, and LA-SCC are summarized in Table 4.
| Levels to be electively irradiated | ||
|---|---|---|
| Tumor types | Ipsilateral node-negative neck | Contralateral node-negative neck* |
| OP-SCC | II, III, IVa, VIIa, (Ib)† | II, (III)‡, (IVa)§, (VIIa)| |
| OC-SCC | Ib, II, III, (Ia)¶, (IVa) | Ib, II, (III)§ |
| HP-SCC | II, III, IVa, Va, (VIb)**, (VIIa)†† | II, III, (IVa)‡‡, (VIb)** |
| LA-SCC | ||
| Supra/subglottis | II, III, IVa, (VIa, VIb)‡‡ | II, III, (IVa)‡‡, (VIa, VIb)§§ |
| Glottis | II, III, (IVa)¶, (VIa, VIb)§§ | II, III, (IVa)‡‡, (VIa, VIb)§§ |
- * Elective contralateral neck irradiation: OP-SCC patients with T3-T4 disease, ipsilateral N2b2-N3 disease, tumors with the body midline involvement, or tumors of midline subsites. OC-SCC patients with ipsilateral N2b2-N3 disease or tumors with the body midline involvement. HP-SCC patients with ipsilateral N2b2-N3 disease, tumors with the body midline, posterior hypopharyngeal wall, or post-cricoid region involvement. LA-SCC patients with ipsilateral N2b1-N3 disease or tumors with the body midline involvement;
- † T4 classification or tumor with oral cavity involvement;
- ‡ T4 classification or ipsilateral N2b1-N3 classification;
- § Ipsilateral N3 classification;
- | T4 classification and ipsilateral N3 classification;
- ¶ T3-T4 classification;
- # T4 classification;
- ** Tumor with esophagus involvement;
- †† Tumor with posterior pharyngeal wall or post-cricoid region involvement;
- ‡‡ T4 classification and ipsilateral N2b2-N3 classification;
- §§ Tumor with subglottis involvement;
- Abbreviations: OC, oral cavity; OP, oropharyngeal; HP, hypopharyngeal; LA, laryngeal; SCC, squamous cell carcinoma.
3.6 An online tool to facilitate the clinical application of our proposals
A publicly available online tool (http://lymph.pvmedtech.com) was constructed to facilitate the clinical use of our elective irradiation recommendations for node-negative necks. For node-positive necks, we referred to the elective irradiation schemes proposed by Gregoire et al. [8]. By inputting the characteristics of the primary tumor and metastatic LNs, elective irradiation schemes for the right and left sides of the neck were recommended and delineated based on a set of computed tomography scans.
4 DISCUSSION
In this study, we established individualized elective irradiation schemes for ipsilateral and contralateral clinically node-negative neck by analyzing the incidence of LNM in patients with OC-SCC, OP-SCC, HP-SCC, and LA-SCC. Our work represents the first study to address the distinct elective irradiation proposals for ipsilateral and contralateral node-negative necks. Our results suggest reduced irradiation volumes for the contralateral neck as opposed to the volumes suggested by guidelines [8, 9], which recommended the same irradiation volumes for bilateral side of the neck. A reduced irradiation volume might help to alleviate radiation-induced toxicities, thus improving the quality of life in cancer survivors [23-25]. Notably, we explored a computer-aided implementation process and constructed a publicly available online tool which might facilitate the widespread clinical use of our proposals.
At present, there are two main guidelines that have reached some consensus for the elective neck irradiation of OC-, OP-, HP- and LA-SCC [8, 9]. Namely, elective irradiation of ipsilateral levels I-III is recommended for the ipsilateral node-negative neck in OC-SCC, and that of ipsilateral levels II-IVa is recommended in patients with OP-, HP- and LA-SCC (T1N0 glottic tumors excluded). For the contralateral neck, the guidelines recommend that the elective irradiation schemes follow the same rules as those for the ipsilateral neck. However, these elective irradiation strategies ignore the impact of T classification on bilateral LNM and the ipsilateral N classification on contralateral LNM, as well as the lower LNM rates in the contralateral neck than those in the ipsilateral neck. Therefore, to draw better proposals, we included these factors in our analysis.
Previous studies have confirmed that the depth of invasion, dimension, and thickness of the primary lesion as well as the T classification were related to regional LNM in HNSCC [26-28]. Lim et al. [29] reported that the occult LNM rate in patients with T3-T4 disease was significantly higher than that in patients with T1-T2 disease (25%–36% vs. 0%–5%). In addition to T classification, the ipsilateral N classification has also been confirmed to be associated with contralateral LNM [30, 31]. Moreover, although the pattern of LNM in the contralateral neck was found similar to that in the ipsilateral neck, the incidence of LNM at certain levels of the contralateral neck was significantly lower than that at the corresponding levels of the ipsilateral neck [13, 14]. Taken together, these suggest that it might be more reasonable to propose distinct elective irradiation schemes for the ipsilateral and contralateral node-negative neck based on the invasion extent of the primary lesion and ipsilateral LNs.
Specifically, for the ipsilateral node-negative neck, our recommended elective irradiation schemes are almost consistent with the guidelines in these four types of cancers, except for some slight differences. First, we suggest that level Ia could be spared in OC-SCC patients with early T classification. Although the guidelines do not distinguish level Ia from level Ib, the low incidence of tumor metastasis in level Ia in our study in patients with T1-T2 disease (0.9%–3.2%) might support the exemption of level Ia in those patients. Second, level IVa could be spared in glottic LA-SCC patients with early T classification because of the paucity of lymphatics in the true vocal cords [15] and the extremely low incidence of LNM to level IVa in patients with T1-T2 disease (0.5%–0.9%). Third, for level Va, since our results and historical data demonstrated that LNM rates were higher than 5% [13, 32], we suggest that level Va might be included in patients with HP-SCC.
For elective irradiation schemes of the contralateral node-negative neck, the guidelines recommend covering levels II-IVa for all patients with OP-SCC, HP-SCC, and LA-SCC (T1N0 glottic tumors excluded). However, our study only suggested level IVa irradiation in patients with advanced ipsilateral N or T classifications, and we regard it as reasonable to spare the lower neck in other patients for the following reasons: (a) our study and historical data [13, 30] consistently demonstrated an extremely low incidence of tumor metastasis to the contralateral level IVa (our study, 0.1%–2.5%; historical data, 0%–5%); (b) in a study published by Kjems et al [33], among 471 patients with OP-SCC who received elective irradiation of the bilateral or ipsilateral levels I-III, only eight patients experienced LN relapse outside the neck irradiation field, and only one case occurred in the contralateral level IV; and (c) sparing the lower neck would reduce radiation doses to the thyroid and might reduce the incidence of long-term hypothyroidism [34, 35]. In addition, we suggest sparing the contralateral level III in most patients with OC- and OP-SCC, except for those with advanced ipsilateral N and T classifications. This is another clinically relevant difference between our proposals and the guidelines for reducing irradiation volumes.
However, because the treatment strategies that patients receive in a real clinical scenario are not consistent with our recommended strategies, it is unknown whether patients would actually benefit from the strategies proposed in this retrospective study. To address this issue, we intend to conduct prospective clinical trials to verify whether our recommended elective irradiation schemes, by which the irradiation volumes were reduced when compared to the guidelines, could improve patients' quality of life without compromising their treatment outcome. Considering that some of the patients in this study did not have pathological results of LNM, we only referred to the imaging results to diagnose the clinically metastatic LNs. We acknowledge this limitation; however, studies have shown good sensitivity and specificity for modern imaging diagnoses of LNM, and imaging is crucial for the diagnosis of LNM at the levels outside the typical neck dissection areas [36-39]. Another limitation is that the majority of patients in this study did not receive pretreatment HPV detection. Therefore, all patients were restaged according to the 7th edition of the UICC/ AJCC staging system.
5 CONCLUSIONS
We proposed individualized and computer-aided elective irradiation schemes for the ipsilateral and contralateral clinically node-negative neck. Our proposals could reduce irradiation volumes compared with those recommended in the current guidelines, and thus, might have a positive impact on patients' quality of life after radiotherapy.
ACKNOWLEDGMENTS
None
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the institutional review board (IRB reference No.: B2020-025-01), and the requirement to obtain informed consent was waived.
CONSENT FOR PUBLICATION
Not applicable
AVAILABILITY OF DATA AND MATERIALS
The datasets during the current study have been deposited in the Research Data Deposit public platform (www.researchdata.org.cn) with the accession code RDDA2020001486 and are available from the corresponding author on reasonable request.
COMPETING INTERESTS
The authors declare no potential conflicts of interest.
FUNDING
This work was supported by the National Natural Science Foundation of China [grant number 81872463 and 81930072], Special Support Program of Sun Yat-sen University Cancer Center [grant number 16zxtzlc06], Key-Area Research and Development Program of Guangdong Province [grant number 2019A1515012045 and 2019B020230002], Health & Medical Collaborative Innovation Project of Guangzhou City, China [grant number 201803040003], Science and Technology Program of Guangzhou, China, [grant number 201607010199], Innovation Team Development Plan of the Ministry of Education (No. IRT_17R110), Overseas Expertise Introduction Project for Discipline Innovation (111 Project, B14035) and Natural Science Foundation of Guang Dong Province (No. 2017A030312003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AUTHOR CONTRIBUTIONS
Study concept and design: JK, LL, YS.
Data acquisition: JK, LL, GQZ, YS.
Data analysis and interpretation: JK, LL, CYJ, MQT, XJ.
Manuscript preparation: JK, LL.
Critical revision: All authors.




