Inhibition of Bone Morphogenetic Protein Signaling Prevents Tau Pathology in iPSC Derived Neurons and PS19 Mice
Abstract
Objective
Many neurodegenerative disorders share a common pathologic feature involving the deposition of abnormal tau protein in the brain (tauopathies). This suggests that there may be some shared pathophysiologic mechanism(s). The largest risk factor for the majority of these disorders is aging, suggesting involvement of the aging process in the shared pathophysiology. We test the hypothesis that an increase in bone morphogenetic protein (BMP) signaling that occurs during aging contributes to the onset and progression of tauopathies.
Methods
Human induced pluripotent stem cell (iPSC)-derived neurons from patients with Alzheimer's disease (AD) were used to investigate the effects of BMP signaling on tau phosphorylation and release and the mechanisms underlying these effects. Wildtype mice were used to examine effects of BMP signaling in vivo. P301S (PS19) mice were examined for the effects of BMP signaling in a model of tauopathy.
Results
Here, we show that BMP signaling, mediated by non-canonical p38 signaling, increases tau phosphorylation and release of p-tau in human iPSC-derived AD neurons. Further, there is an interaction between BMP signaling and apolipoprotein E4 (ApoE4) that significantly increases tau phosphorylation and release compared with ApoE3 neurons. Inhibiting BMP signaling reduces the changes in tau in the cultured human neurons, and it limits tau pathology and prevents cognitive decline in PS19 mice.
Interpretation
Our study suggests that the age-related increase in BMP signaling may participate in the onset and progression of tau pathology. Thus, therapeutic interventions that reduce BMP signaling in the aging brain could potentially slow or prevent development of diseases involving tau hyperphosphorylation. ANN NEUROL 2025;97:657–672
Various neurodegenerative disorders, including frontotemporal dementia (FTD), Alzheimer's disease (AD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and others, share a common pathologic feature involving the deposition of abnormal tau protein in the brain (tauopathies).1-3 This implies that there may be some shared pathophysiologic mechanism(s). The primary risk factor for nearly all of these conditions is aging, implying that the aging process plays a role in their common pathophysiology.4, 5 Genetic risk factors also influence the incidence of tauopathies.6 For example, the apolipoprotein e4 allele (APOE4) is the most significant genetic risk factor for developing AD, and individuals with this allele demonstrate increased phosphorylated tau (p-tau) and amyloid burden, increased brain atrophy, and amplified progression of the disease.7-9 APOE4 is also a lesser risk factor for FTD, and it lowers the age of onset of this disease.10, 11 Thus, both aging-related changes and genetic background influence disease onset and progression.
Aging is associated with biochemical and morphological changes in the brain that may shed insight into the age-related susceptibility to tauopathies. In particular, changes in hippocampal structure and connectivity are highly associated with aging.12 We and others have demonstrated that bone morphogenetic protein 4 (BMP4) levels increase with age in the dentate gyrus of the mouse hippocampus, whereas levels of the BMP inhibitor, noggin, decrease.13-16 A similar age-related increase in BMP signaling is found in the human brain and other BMP ligands are higher in AD brains than in age-matched controls.17, 18 Interestingly, higher levels of BMP signaling are associated with worse cognitive performance in adult and aged mice.13 Moreover, reducing hippocampal BMP signaling in aged mice reverses aging-related changes in hippocampal neurogenesis and cognition,13, 16 which suggests that increased BMP signaling underlies some aging-related changes in hippocampal function.
The precise mechanisms by which genetic risk factors of AD, such as APOE4, interact with the aging process to initiate disease or cognitive decline are unknown. However, APOE expression is regulated by BMP signaling in organs outside the brain,19 and BMP signaling exacerbates human disorders associated with APOE dysfunction, including diabetes, atherosclerosis, and hepatic steatosis.20, 21 Signaling through the p38 mitogen-activated protein kinase (MAPK), a downstream mediator of non-canonical BMP signaling, has been implicated in APOE4-driven pathology in the brain.22 These observations suggest a possible point of convergence between aging-related increases in BMP signaling and APOE4-associated disease pathogenesis. In this study, we find that enhanced BMP signaling increases tau phosphorylation in an APOE4-dependent manner, and that limiting the age-related increase in BMP signaling prevents the onset of tauopathy in a mouse model of FTD.
Materials and Methods
Experimental Model and Subject Details
Human Pluripotent Stem Cells
Fibroblasts from patients with AD (AD1 = AG011414; AD2 = AG05810, and AD3 = AG04402) were reprogrammed and CRISPR Cas9 edited from E3/E4 to E3/E3, as previously described.23 Confirmation of APOE genotypes and identification of mutations in known AD-associated genetic loci were performed by whole genome sequencing (Novogene). AD1 contains a presenilin 1 (PS1) mutation and is categorized as a familial AD line, whereas AD2 and AD3 do not contain protein modifying mutations in amyloid precursor protein and presenilins 1/2 and are therefore considered sporadic AD lines. The iPSCs were cultured on Matrigel (Corning, 08-774-552) with mTeSR1 (Stemcell Technologies, 85850). The iPSC lines were maintained in an incubator at 37°C with 5% CO2 incubator.
Differentiation of iPSCs into Neurons and BMP4 Treatment
Generation of APOE 3/4 and CRISPR/CAS9 corrected isogenic APOE 3/3 AD (AD1 = AG11414, G10; AD2 = AG05810, D6; and AD3 = AG04402; H7) were previously described and obtained from Coriell Institute for Medical Research or Northwestern University.23 The iPSCs were differentiated into forebrain excitatory neurons via lentiviral-mediated overexpression of neurogenin-2, as previously described with minor modifications.24 Stem cells were maintained in mTeSR1 media on Matrigel-coated 6-well tissue culture plates (Corning, 353046). Upon the start of differentiation, cells were single cell dissociated using Accutase (Millipore) and resuspended in mTeSR1 with 10 uM ROCK Inhibitor, Y-27632 (Reprocell, 04-0012-02), with lentiviruses encoding rtTA and pTetO-Ngn2-puro (generated by NU SBDRC). The following day, the medium was changed to KO-DMEM (Thermo Fisher Scientific, 10829018), 1X MEM nonessential amino acids (Life Technologies, 11140-050), 1X Glutamax (Thermo Fisher Scientific, 35050-061), and 0.1% 2-mercaptoethonal (Gibco, 21985023), supplemented with 10 uM SB431542 (Stemgent, 04-0010-05), 100 nM LDN193189 (Stemgent, 04-0074-02), 2 uM XAV939 (Tocris Bioscience, 3748), and 3 ug/ml doxycycline (Sigma, D3072). Gradually, the medium was changed over 2 days to neural induction medium, DMEM/F-12 containing MEM nonessential amino acids, Glutamax, N-2 (Invitrogen, 17502-048), D-glucose (Sigma-Aldrich, G8769), 2 ug/ml heparin sulfate (Sigma, H3149) supplemented with 3 ug/ml doxycycline and 2 ug/ml puromycin (Sigma, P9620). Induced neurons were replated onto 6-well tissue culture plates at 450 k cells/well on pre-coated with poly-L-orthnithine (PLO) (Sigma, P4957), 4 ug/ml Laminin (Roche, 11243217001), and 2 ug/ml Fibronectin (Sigma, F1141), cultured with neuronal maturation medium (BrainPhys Basal Medium [Stemcell Technologies, 05791], B-27 [Thermo Fisher Scientific, 17504044] and N-2 supplements [Thermo Fisher Scientific], MEM nonessential amino acids, and Glutamax, supplemented with 3 ug/ml doxycycline and 10 ng/ml BDNF [Peprotech, AF-450-02]). Post-plating, cells were treated with 3 uM Ara-C (Thermo Fisher Scientific, 449561000) for 48 hours to eliminate proliferating cells. Half medium was exchanged every 2 to 3 days until cell collection. The human induced pluripotent stem cell (iPSC)-derived neurons (iNs) were treated with 10 ng/ml BMP4 (R&D systems, 314-BP) or 250 ng/ml noggin (Peprotech, 120-10C-20) at 35 days post induction for 72 hours followed by collection for analysis.
Mice
All animal procedures and experiments followed the Public Health Service Policy on Humane Care and Use of Laboratory Animals and under guidelines approved by the institutional Care and Use Committee (IACUC) at Northwestern University Feinberg School of Medicine. All experiments were conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Animals were housed 3 to 5 animals per cage on a standard 14:00 hour light:dark cycle in a temperature-controlled environment, having access to food and water ad libitum. All behavioral testing was conducted during the light cycle.
The P301S (PS19) Tau transgenic mice expressing 1N4R tau overexpressing the human P301S tau mutation were purchased from Jackson Laboratories (Stock No. 008169). Mice were bred hemizygous to non-carrier to avoid the lethal death of homozygous offspring. Wildtype littermates were used as control animals to account for any background effects. Wildtype mice (8–10 weeks old), used for BMP4 stereotaxic injections, were ordered from Jackson Laboratories. The generation of NSE-BMP4 mice was previously described.25 In short, the NSE-BMP4 transgene was constructed by cloning a 1246-bp fragment containing the murine BMP4 cDNA downstream of the rat neuron-specific enolase (NSE) promoter and upstream of an SV40 polyadenylation signal.26 The NSE promoter construct contained the initial, noncoding exon of the rat NSE locus, which increases expression levels. The transgene fragment was isolated and injected into C57Bl/6 mouse embryos, and founder animals were identified by Southern blot analysis according to a standard protocol. Three stable lines were obtained, and these were maintained by successive backcrosses to C57Bl/6.27 Mice were genotyped through polymerase chain reaction (PCR) using genomic DNA and transgene-specific primers (Transnetyx). All experiments were performed with both sexes, and control groups used were littermates, controlling for background and environment. All experiments and data analysis were conducted by researchers blinded to the genotype or treatment groups of mice.
Method Details
Stereotaxic Injections
Direct bilateral dentate gyrus injections of lentivirus expressing either noggin or BMP4 were performed using a mouse stereotaxic instrument (Stoelting, Wood Dale, IL, USA), a Prototypical Stereotaxic Injector (Stoelting, Wood Dale, IL, USA), and a 10 ul Hamilton micro syringe. Mice were anesthetized using isoflurane inhalation and sterilely prepped.13, 28 A midline scalp incision was made, and 2 tiny craniotomies were performed over the hippocampus of each hemisphere. Then, 2 ul of virus were injected bilaterally into the dentate gyrus at a rate of 0.5 ul/min at the following coordinates relative to bregma: 2 mm posterior, 1.5 mm lateral, and 1.9 mm ventral. Lentiviral infection was confirmed by immunohistochemistry (Fig. 4B), and mice lacking successful virus infection in the bilateral dentate gyrus were excluded from the analysis. For BMP4 overexpression experiments, the mice were perfused and analyzed for hippocampal p-tau (AT8) protein 3 weeks after the viral injections. For noggin overexpression experiments, the mice were undisturbed in their home cage for approximately 6 months post-injection until they reported tau pathology.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Cultured neurons were dissociated into single cells using accutase (Stemcell Technologies, 07920) for 10 minutes prior to quenching with media. Cells were transferred to a 15 ml Falcon conical tube (Corning, 352070) and spun at 0.8 RPM for 5 minutes. Cells were resuspended in cold 1x PBS (Corning, 21-040-CV). RNA was extracted using RNAqueous Micro Total RNA isolation Kit (Thermo Fisher Scientific, AM1931) according to the manufacturer's instructions. All RNA samples were subjected to DNAse digestion provided by the kit and eluted in 25 ul of ultra-pure H2O. RNA quality control was performed with Nanodrop 2000c spectophotometer. Reverse transcription was performed using SuperScript IV VILO Master Mix (Thermo Fisher Scientific, 11766050) following the manufacturer's instruction. The quantitative PCR (qPCR) was performed using SYBR Green PCR Master Mix (Thermo Fisher Scientific, 4309155) using MicroAmp Optical 384-well reaction plates (Thermo Fisher Scientific, 4309849) QuantStudio 7 Real-Time PCR machine through the NUseq core at Northwestern University. DNA sequence of primers used for pPCR can be found in Table 1. For analysis of qPCR data, we assessed the relative expression of BMP signaling genes in APOE3 and APOE4 neurons. GAPDH was used as the housekeeping gene. Threshold cycle (Ct) values obtained from for GAPDH and target genes were used to calculate the ΔCt values, which were compared to the ΔCt value of APOE3 in neurons to determine the ΔΔCt. The relative levels were expressed as fold changes.
| Primer | Sequences |
|---|---|
| BMP2 | ACCCGCTGTCTTCTAGCGT |
| TTTCAGGCCGAACATGCTGAG | |
| BMP4 | ATGATTCCTGGTAACCGAATGC |
| CCCCGTCTCAGGTATCAAACT | |
| BMP6 | AGCGACACCACAAAGAGTTCA |
| GCTGATGCTCCTGTAAGACTTGA | |
| BMP7 | TCGGCACCCATGTTCATGC |
| GAGGAAATGGCTATCTTGCAGG | |
| NOG | CCATGCCGAGCGAGATCAAA |
| TCGGAAATGATGGGGTACTGG | |
| FSTN1 | GAGCAATGCAAACCTCACAAG |
| CAGTGTCCATCGTAATCAACCTG | |
| FSTN4 | TCCTGGGAAAGAGGATCACCG |
| TCTGCATCTAAGTCCCTGAACA | |
| P21 | TGTCCGTCAGAACCCATGC |
| AAAGTCGAAGTTCCATCGCTC | |
| ID1 | CTGCTCTACGACATGAACGG |
| GAAGGTCCCTGATGTAGTCGAT | |
| ID3 | GAGAGGCACTCAGCTTAGCC |
| TCCTTTTGTCGTTGGAGATGAC | |
| ALK1 | CGAGGGATGAACAGTCCTGG |
| GTCATGTCTGAGGCGATGAAG | |
| EGR1 | GGTCAGTGGCCTAGTGAGC |
| GTGCCGCTGAGTAAATGGGA | |
| TGFBR1 | ACGGCGTTACAGTGTTTCTG |
| GCACATACAAACGGCCTATCTC | |
| MAPT | CCAAGTGTGGCTCATTAGGCA |
| CCAATCTTCGACTGGACTCTGT | |
| GAPDH | GGAGCGAGATCCCTCCAAAAT |
| GGCTGTTGTCATACTTCTCATGG |
- qRT-PCR = quantitative real-time polymerase chain reaction.
Bulk RNA-Sequencing
Cultured neurons were generated in 3 independent differentiation experiments from 2 pairs of isogenic iPSC lines using NGN2 excitatory neuron differentiation protocol. At 35 days in vitro (DIV), neurons were dissociated into single cells using accutase. RNA was extracted from isolated cells using the RNAqueous Micro total RNA kit, according to manufacturer's instructions. Samples with a Bioanalyzer RNA integrity number greater than 8 were used for sequencing. Sequencing was performed by The University of Chicago Genomic facility and the data analyzed by the Northwestern University Sequencing Core bioinformatic team. Specifically, fastQC version 0.12 and multiQC version 1.18 were used for quality control of raw sequence data, followed by mapping to the human reference genome (hg38) using STAR2.7.2. Reads were quantified to genomic features using featureCounts version 1.5.3 and Subread package with gene annotation included in GENCODE release 31. Expression normalization were performed using TMM normalization with library size correction, followed by differentiation gene expression analysis performed by DEseq2 R package. Processed expression data is deposited at GEO repository at accession number: GSE253647.
Protein Extraction and Immunoblotting
Cellular proteins were collected using M-PER (Thermo Fisher Scientific, 78505) supplemented with 1 mM Halt™ protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, 78444). For complete protein isolation, lysate was kept on ice for 30 minutes and vortexed occasionally followed by 20 minutes 14.3 revolutions per minute (RPM) centrifugation at 4°C. Protein quantification was performed with Rapid Gold BCA reagents (Thermo Fisher Scientific, A53226) using a Biotek Synergy HTX microplate reader. Protein was run on 4% to 20% tris-glycine gels (Bio-Rad Laboratories, 4561085) and transferred to Immobilon polyvinylidene difluoride membranes (PVDF; Millipore, IPVH00010). The membranes were blocked in 5% BSA for pSMAD1/5/8 (Millipore, AB3848), BMP4 (Origene, UM500038), and Noggin (R&D Systems, AF719-SP). For all other immunoblots, membranes were blocked in 5% Blotto (Santa Cruz, 2325) in Tris-buffered saline (Bio-Rad Laboratories, 1706435). Primary antibodies were rabbit anti-APOE (Cell Signaling Technology, 7074S), mouse anti-total tau (HT7; Thermo Fisher Scientific, MN1000), mouse anti-ptau (AT8; Thermo Fisher Scientific, MN1020), rabbit anti-LC3 (Sigma-Aldrich, L8918), rabbit anti-P38 (Cell Signaling Technology, 9211S), rabbit anti-phospho-P38 (Cell Signaling Technology, 9211S), and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz, sc-365,062). Secondary antibodies were horse anti-mouse horseradish peroxidase (HRP; Cell Signaling Technology, 7076) and goat anti-rabbit HRP (Cell Signaling Technology, 7074). Membranes were incubated with Radiance Q Plus chemiluminescent substrate (Azure Biosystems, AC2101) and developed on the Azure c600 bioanalytical imaging system. Quantification of all blots was performed with ImageJ (NIH, Bethesda, MD, USA). A consolidated list of antibodies can be found in Table 2.
| Reagent or resource | Source | Catalog |
|---|---|---|
| Antibodies | ||
| BMP4 | Origene | AM01363PU-N |
| Noggin | Origene | TA500028 |
| AT8 | Thermo Fisher Scientific | MN1020 |
| HT7 | Thermo Fisher Scientific | MN1000 |
| Psmad 1/5/8 | EMD Millipore | AB3848- |
| Anti-B-Amyloid [6e10] | BioLegend | 803,014 |
| Phospho p38 | Cell Signaling | 9211S |
| p38 | Cell Signaling | 9212S |
| PSD-95 | Thermo Fisher Scientific | MA1-046 |
| Cleaved Cas3 | Cell Signaling | 9,661 |
| GFAP | DAKO | GA524 |
| Bacterial and virus strains | ||
| NGN2 | Zhang et al | |
| RTTA | Zhang et al | |
| Chemicals, peptides, and recombinant proteins | ||
| Y27632 | Reprocell | #04-0012-02 |
| LDN193189 | Stemgent | #04-0074-02 |
| SB431542 | Stemgent Reprocell | #04-0010-05 |
| BDNF | Peprotech | #AF-450-02 |
| Doxycycline Hydrochloride | Sigma-Aldrich | #D3072-1ML |
| Puromycin dihydrochloride | Sigma-Aldrich | #P9620-10ML |
| Cytosine beta-D-arabinofuranoside | Sigma-Aldrich | #C1768-100MG |
| BMP4 | R&D Systems | #314-BP CF |
| Noggin | Peprotech | #120-10C-20 |
| SB203580 | Sigma-Aldrich | #S8307-1MG |
| Experimental models: Cell and mouse lines | ||
Human induced pluripotent stem cell Line: AG02261 |
Duan et al | |
Human induced pluripotent stem cell Line: AG011414 |
Wadhwani et al | |
Human induced pluripotent stem cell Line: Clone G10 |
Wadhwani et al | |
Human induced pluripotent stem cell Line: AG05810 |
Wadhwani et al | |
Human induced pluripotent stem cell Line: Clone D6 |
Wadhwani et al | |
Human induced pluripotent stem cell Line: AG04402 |
This paper | |
Human induced pluripotent stem cell Line: Clone H7 |
This paper | |
| Oligonucleotides | ||
| Primers for qPCR, see Table 1 | This paper | NA |
| Software and algorithms | ||
| ImageJ | ||
| Prism | ||
| Cell Profiler | ||
| Enricher | ||
- NA = not applicable; qPCR = quantitative polymerase chain reaction.
Enzyme-Linked Immunosorbent Assay
Neurons were treated with BMP4 (10 ng/ml) or vehicle and incubated for 72 hours to accumulate secreted proteins in media. Conditioned media was collected and treated with Halt protease and phosphatase inhibitor cocktail and centrifuged to remove any cellular debris. Secreted levels of proteins of interests were quantified using the following enzyme linked immunoassays: APOE (Cell Biolabs, STA-367), p-tau p181 (RayBiotech, PEL-Tau-T181-Q-1), p-tau PHF-1 (Biomatik, EKF57692-96T), total tau (Invitrogen, KHB0041), and Abeta 40/42 (Thermo Fisher Scientific, KHB3441 and KHB3481). Kits were performed per the manufacturer's instructions.
Immunohistochemistry
Mice were transcardially perfused with 1X PBS before the brains were dissected and divided into 2 hemispheres by the midline. One hemisphere was fixed in 4% paraformaldehyde for 2 hours followed by subsequent preservation in 30% sucrose for 24 hours. Ten micrometer thick sections were obtained using a Leica CM3050s cryostat, and mounted on Superfrost Plus microscope slides (Fisher Scientific, 12-550-15). Slides were outlined with a hydrophobic barrier pen for staining (Vector laboratories, H400). Sections were washed 3 times in PBS, blocked in 10% normal goat serum with 0.25% Triton X-100 in PBS for 1 hour at room temperature (RT), and incubated overnight in primary antibody diluted in 3% normal goat serum with 0.25% Triton X-100 in PBS at 4°C. After primary incubation, the slides were washed 3 times with PBS, following fluorophore conjugated-secondary antibody (Alexa-488, Alexa-555, or Alexa-647, Thermo Fisher) and Hoechst 33342 (Thermo Fisher Scientific, 62249) for nuclear stain incubated for 1 hour at RT. After secondary incubation, the slides were washed 3 final times with PBS and slides were coverslipped with Prolong Diamond Antifade Reagent (Thermo Scientific, P3970). Primary antibodies used were chicken anti-mCherry (Origene technologies, TA150127), mouse anti-AT8 (Thermo Fisher, MN1020), GFAP (Dako, Z033401), rabbit anti-pSMAD 1/5/8 (Millipore, AB3848), rabbit anti-pP38 MAPK (thr180/try182; Cell Signaling, 9211S), goat anti-NeuroD1 (Invitrogen, PA5-47381), and rat anti-sox2 (Invitrogen, 14-9811-80).
Confocal Imaging and Quantification
Images were attained using either a Leica SP5 Confocal Microscope or Keyence fluorescence BZ-X810 microscope. For confocal imaging, Z-stacks of the dentate gyrus were obtained (step size = 2 uM) using sequential scanning to prevent bleed through between channels. Three or more Z-stacks of equal thickness and equivalent positions were quantified for each brain. Cell counts were normalized per volume of the dentate gyrus granule cell layer using ImageJ software. All imaging and quantification were performed blinded to experimental groups and treatments.
Behavioral Testing
Barnes Maze
A modified Barnes maze protocol was executed as described.29 A circular white platform (90 cm in diameter) with 20 holes (5 cm diameter) arranged around the edge of the platform was mounted 80 cm above the floor. Each hole is equally spaced, with 1 hole indicated as the target with a hidden escape box. The platform was surrounded by spatial cues at the height of the maze to aid in locating the target escape box. Every day before performing the task, the mice were acclimated to the testing room for an hour. The animals were subjected to the Barnes mask task in 3 stages: (1) habituation day (d1), (2) training day (d2-4), and (3) probe day (d5). On the habituation day, each animal was placed in the center of the maze and allowed to explore the platform for 120 seconds. On training days, each animal was placed in the center of the maze and allowed to explore the platform for 300 seconds. For both habituation and training days, the trial ended if the animal found the escape box and entered it. If the animal could not find and enter the escape box after the trial time, it was gently guided to the escape box. A white noise was played during each trial while the mouse explored the platform, searching for the escape box. Once the mouse entered the escape box, either by itself or by guidance, the white noise was paused, and the mouse was kept in the escape box for 60 seconds. This was repeated for all 3 training days, consisting of 2 daily trials to enforce the target platform location. On probe day, the escape box was replaced with a false box with no entrance, and the animal was allowed to explore the platform for 180 seconds. Primary latency, total latency, total distance, and total speed were measured using WaterMaze software. Heat map analysis was performed using AnyMaze.
Y-Maze
The spontaneous alteration Y-maze test was implemented as previously described.13, 30 In brief, the mouse was placed in a chamber containing 3 radial arms, 60 cm long, 14 cm wide, and 18 cm in height. The mice explored the chamber for 8 minutes. Spontaneous alterations were calculated as the percentage of entry triads (entries into each of the 3 arms consecutively) to the total number of possible triads. Limelight software (version 6) was used for data analysis.
Statistical Analyses
Data were analyzed using Prism 10 software (GraphPad) and all results were reported as a mean ± SEM. Analysis of 2 groups was conducted using the Student's unpaired t test under normal distributions. Statistical analysis of more than 2 groups was performed using ordinary 1-way analysis of variance (ANOVA) followed by a Tukey's post hoc test under normal distribution. Discrimination analysis was associated between groups and analyzed using the Student's unpaired t test. Statistical significance was predetermined and assigned at the cutoff of p < 0.05. The exact sample size for each experiment is represented in each figure.
Results
Increased BMP Signaling Alters Tau Phosphorylation and Secretion in iPSC-Derived Neurons
To examine the potential role of BMP signaling in tauopathy, we first asked whether increased BMP signaling promotes tau phosphorylation in vivo. To address this question, we used 2 different mouse models. In the first model, we stereotaxically injected a lentivirus (LV) expressing either BMP4 (LV-BMP4) or m-Cherry (LV-control) into the dentate gyrus of the hippocampus of wild-type mice. Three weeks later, the hippocampus was examined immunohistochemically for p-tau levels using the p-tau-specific AT8 antibody (Fig 1A). Overexpression of BMP4 resulted in significantly increased levels of p-tau in both the granular cell layer and hilus of the dentate gyrus (Fig 1B). In the second model, we used a transgenic mouse line where overexpression of BMP4 is achieved under the control of the neuron-specific enolase promoter (NSE-BMP431; Supplementary Fig S1A). These animals have systemic overexpression of BMP4 throughout their lives. We similarly found significantly increased levels of p-tau in the dentate gyrus of these animals (Supplementary Fig S1B).25, 32 These observations suggest that the age-related increase in BMP signaling in the human hippocampus could potentially initiate or accelerate the development of tauopathy.
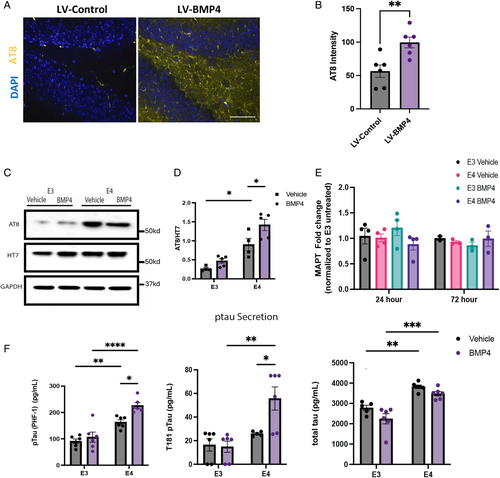
We therefore utilized human induced pluripotent stem cell (iPSC)-derived neurons (iNs) to examine the effects of BMP signaling on tau phosphorylation. The p-tau burden and the rate of disease progression are greater in patients with the APOE4 genotype compared to APOE3, and we have previously shown increased phosphorylation and secretion of tau by iNs in an APOE4-dependent manner.23 We, therefore, asked whether this might reflect, at least in part, differences in responses to BMP signaling between human APOE3 and APOE4 neurons. The iPSCs derived from 3 different patients with AD with an APOE4 genotype were CRISPR/CAS9 corrected to the APOE3 genotype.23 The APOE4 (E4) and APOE3 (E3) isogenic iPSC cells were differentiated into induced excitatory neurons (iNs),24 and were examined by Western analysis for levels of p-tau and total tau after treatment with BMP4 or vehicle (Fig 1C). Levels of p-tau in vehicle-treated cells were significantly higher in E4 neurons compared with E3, consistent with prior studies (Fig 1D). Further, treatment with BMP4 resulted in a significant increase in levels of p-tau in E4 neurons but only minor and non-significant increases in isogenic E3 iNs. By contrast, total tau levels were comparable in E3 and E4 iNs. Similarly, levels of tau mRNA were comparable and not altered by treatment with BMP4 (Fig 1E). Secretion of p-tau has been hypothesized to be responsible for the transsynaptic spread of AD33 in the characteristic pattern described by Braak et al.34 We therefore measured levels of secreted p-tau and total tau and found that media from E4 neuron cultures contained higher levels of p-tau and tau than media from E3 neuron cultures (Fig 1F). Moreover, BMP4 treatment significantly increased the secretion of p-tau by E4 neurons (Fig 1F). Because abnormalities in amyloid processing are a hallmark feature of AD, we also examined the effects of BMP signaling on levels of amyloid β fragments after BMP treatment (Supplementary Fig S1C, D). Cellular levels of amyloid β were consistent across both E3 and E4 iN, regardless of the treatment condition. In addition, secreted levels of neither amyloid β40 nor amyloid β42 were changed by BMP4 treatment (Supplementary Fig S1E), and no differences were observed between genotypes or treatments. Thus, BMP signaling influences both tau phosphorylation and p-tau secretion, but not amyloid accumulation or processing, in an APOE4-regulated manner.
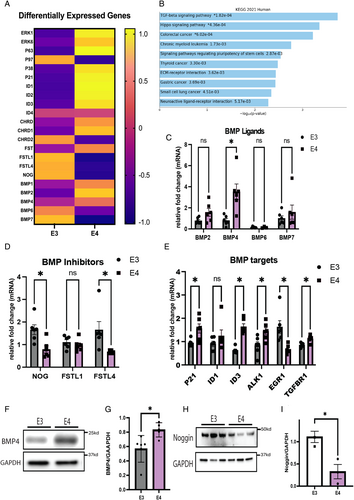
Differential Expression of BMP Family Members Between APOE3 and APOE4 iNs
We then asked whether differences in the APOE genotype conversely alter BMP signaling. Bulk RNA sequencing (RNAseq) analysis was performed to evaluate baseline transcriptomic differences between APOE3 and APOE4 iNs. Differential gene expression analysis of known components of the BMP signaling pathway revealed higher levels of both BMP ligands and downstream activators in E4 iN (Fig 2A). In addition, pathway enrichment analysis revealed that transforming growth factor β (TGFβ) signaling (which includes BMP signaling), extracellular matrix (ECM)-receptor interactions, neuroactive ligand-receptor interactions, and the hippo signaling pathway are all relatively increased in E4 compared with E3 iNs (Fig 2B), with the TGFβ signaling pathway identified as the most significant different gene ontology term between E3 and E4 iNs based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Validation of the bulk RNAseq findings was performed on select BMP ligands and receptors by qPCR, which demonstrated similar increases in BMP4 mRNA in E4 iNs compared with E3 iNs (Fig 2C). Higher but not statistically different levels of BMP2 and BMP7 mRNA were also detected in E4 iNs compared with E3 iNs, whereas BMP6 mRNA was not detected at high levels in iNs and its expression was not different between E3 and E4 iNs. BMP signaling is regulated by proteins such as noggin and follistatin that bind and sequester BMP ligands away from receptors, thereby inhibiting BMP signaling.35 The qPCR also confirmed that both noggin and follistatin like-4 mRNA levels were significantly lower in E4 compared with E3 iNs, whereas follistatin like-1 levels did not differ between groups (Fig 2D). The mRNA levels of direct BMP/pSMAD target genes ID3 and CDKN1A (P21) were also higher in E4 iNs, and ID1 levels trended higher (Fig 2E). Western blot analyses of BMP4 and noggin protein in E3 and E4 iNs revealed changes that parallel those observed at the mRNA level (Fig 2F–I). Taken together, these findings suggest that APOE genotype differences differentially impact expression of BMP signaling-associated genes.
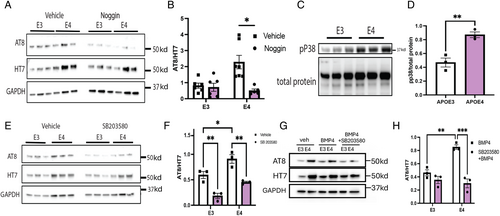
Mechanisms Mediating the Effects of BMP Signaling on APOE Expression and Tau Phosphorylation
Because we found that BMP signaling increases levels of p-tau, the decrease in levels of transcripts for BMP inhibitors and the increase in transcripts for BMP ligands in E4 iNs suggest that the increased levels of p-tau in E4 iNs might reflect elevated endogenous BMP signaling. In fact, we found that treatment of E4 iN with noggin reduced p-tau levels to levels comparable with E3 iNs (Fig 3A, B), demonstrating the role of endogenous BMP signaling in the regulation of p-tau. We next asked what mechanisms mediate the effects of BMP signaling on tau phosphorylation. In addition to phosphorylation of the canonical BMP signaling target SMAD1/5/8, downstream effectors of BMP signaling also include a number of mitogenic kinases,36 including pP38, pERK1/2, pGSK, and pCDK5 (Supplementary Fig S2A). Comparison of phospho-SMAD (Supplementary Fig S2B, C), phospho-p38 (pp38; Fig 3C, D), and phospho-ERK1/2 (Supplementary Fig S2B, E) protein expression by western blots revealed higher levels in E4 iNs relative to E3 iNs. Treatment with PD0325901, an inhibitor of ERK1/2, did not alter levels of p-tau (Supplementary Fig S2F, G), but treatment with SB203580, an inhibitor of p38, reduced levels of p-tau in both E3 and E4 iNs (Fig 3E, F). We, therefore, asked whether p38 mediates the increase in p-tau in E4 iNs treated with BMP4 and found that the p38 inhibitor prevented the increase in p-tau in response to BMP4 (Fig 3G, H). In toto, these observations indicate that p38 signaling mediates effects of BMP signaling on tau phosphorylation.
Inhibition of BMP Signaling Improves Hippocampus-Dependent Cognition in PS19 Mice
The profound effects of BMP signaling on tau phosphorylation in cultured human iNs suggest that inhibition of the pathway in vivo might prevent or ameliorate tauopathy. We directly tested this hypothesis in vivo using a well-characterized mouse model of tauopathy, P301S (PS19) transgenic mice, that start showing synaptic deficits at 2 months of age, accumulation of p-tau at 5 months of age, and cognitive deficits as early as 6 months of age.37, 38 The dentate gyrus of 2-month-old PS19 mice was stereotaxically injected with lentivirus expressing either both noggin and mCherry (PS19-nog) or mCherry alone (PS19-control; Fig 4A). We examined the hippocampus for expression of mCherry to validate that the stereotactic injections were correctly targeted to the dentate gyrus of the hippocampus. As shown in Figure 4B, both blades of the dentate gyrus were correctly targeted in both the PS19-control and PS19-nog mice. This age was chosen to precede the onset of p-tau accumulation, disease pathology, and cognitive decline.
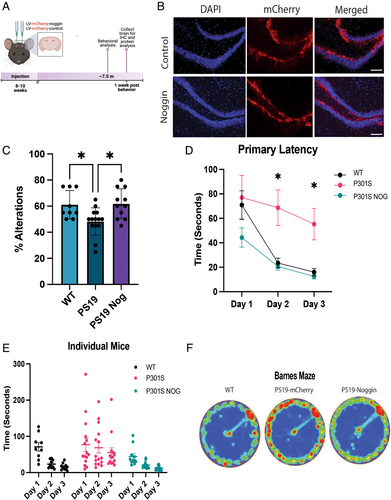
Cognitive function was examined at 7.5 months of age using alternating Y-maze and Barnes Maze (Fig 4C–F). In alternating Y-maze, LV-control mice entered the alternate arm of the maze about 60% of the time (Fig 4C). By contrast, PS19-control entered the alternate arm about 50% of the time, no different than what would occur by chance. PS19-nog mice entered the alternate arm about 60% of the time, which was identical to the performance of wild-type mice. In order to measure learning capacity in these mice, the Barnes maze was utilized. We tested the mice across a 3-day period using primary latency, defined as the time required for mice to find the escape hole in the task. On the first day, before the mice had learned the task, wild-type, and PS19-control mice required the same amount of time to escape from the maze. However, over the next 2 days, the wild-type mice learned to escape much more rapidly, whereas learning was significantly delayed for the PS19-control mice. This indicates a significant cognitive decline in the PS19-control mice at 7.5 to 8.5 months of age, consistent with prior studies.37 Remarkably, the PS19-nog mice performed slightly better on day 1 than the other groups, consistent with the known effects of noggin in enhancing hippocampus-dependent cognition,13, 39 and performed identically to wild-type mice on days 2 and 3. These results show that noggin overexpression completely prevented the cognitive decline in PS19 mice as measured by the Barnes maze testing. As shown in Figure 4D, after training (day 3), wild-type mice spent most of their time (red in Fig 4F) around the escape route. By contrast, PS19-control mice spent time in virtually all quadrants of the maze with only a slight increase in time at the escape area. Remarkably, the exploratory pattern of PS19-nog mice was indistinguishable from wild-type mice, indicating a complete rescue of function. There were no significant differences between groups in the distance traveled and average speed, indicating no issues in motor function (Supplementary Fig S3A–D). Probe day heat map (Supplementary Fig S3E) showed movement of mice once the escape box was removed, as well as the percent of time spent in the target quadrant and target zone (Supplementary Fig S3F, G). Although the time spent on probe day near the target was not significant between groups, the wild-type and PS19-noggin animals were more similar than PS19-mcherry. Overall, cognitive function, as measured by 2 different behavioral tests, was prevented in PS19 mice by overexpression of noggin.
Overexpression of Noggin Reduces Tau Phosphorylation in the PS19 Mice
We next examined the brains of the mice to determine whether overexpression of noggin in the dentate gyrus prevented the accumulation of p-tau and the pathological changes characteristic of PS19 mice compared with control injected and age-matched wild-type mice. pSMAD1/5/8 and BMP4 ligand was upregulated in the dentate gyrus of aged PS19 mice compared with age-matched wild-type controls, indicating increased BMP signaling in this model of tauopathy (Fig 5A–C). We further validated our model by checking levels of pSMAD1/5/8 in the dentate gyrus by Western blot and immunohistochemical analysis of pSMAD positive cells to determine whether noggin overexpression inhibited BMP signaling and found a marked reduction in BMP signaling (Supplementary Fig S4A–C). We evaluated p-tau accumulation in the dentate gyrus immunohistochemically (AT8 antibody; Fig 5D, E). Quantitation of AT8 staining per unit area in the dentate gyrus hilus and granule cell layer regions showed a significant 85% reduction in the PS19-nog brain compared to the PS19-controls. There was a marked reduction in levels of p-tau with only a slight change in total tau in the PS19-nog mice. Levels of p-tau (AT8 antibody) and total tau (HT7 antibody) were then examined by Western analysis (Fig 5F, G). Quantitation of the ratio of AT8 to HT7 showed an almost 50% reduction in tau phosphorylation at the ser202/thr205 sites in the dentate gyrus of PS19-nog mice. To help define the relationship between the anatomic and behavioral effects, we correlated behavioral scores with the AT8/HT7 ratios for individual mice and found a highly significant correlation between behavioral and anatomic rescue (Fig 5H, I). Specifically, the percent alternation in the Y-maze test correlated highly (p = 0.0026) with the AT8/HT7 ratio (Fig 5H), and the primary latency in the Barnes maze test correlated negatively with the ratio (p = 0.0497; Fig 5I).
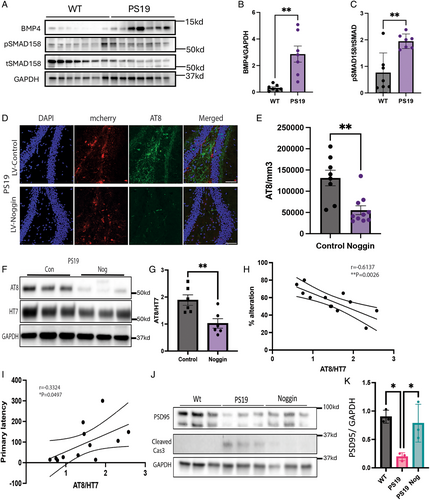
Loss of synapses with an associated loss of synaptic proteins is also characteristic of the pathology of PS19 mice, so we examined levels of the synaptic protein, PSD95, by Western analysis. Levels of PSD95 were significantly higher in wild-type and PS19-nog compared with PS19-control mice (Fig 5J, K). There was also a marked increase in cleaved caspase protein in PS19 mice compared with noggin-treated PS19 mice and wild-type mice, indicating higher levels of apoptosis (Fig 5J). In addition to neuronal death in PS19 mice, gliosis begins early in the PS19 disease trajectory. Inhibition of BMP signaling lowered GFAP expression in both the dentate gyrus and CA3 regions (Supplementary Fig S4D, E), as well as pP38 expression in the CA1 region (Supplementary Fig S4F, G). Last, we probed for neurogenesis/proliferation markers NeuroD1 and Sox2 to evaluate the addition of adult born neurons induced by inhibition of BMP signaling (Supplementary Fig S4H). There were significant increases in sox2 in the SGZ indicating increased numbers of the neural stem cell niche (Supplementary Fig S4I). Subsequently, there were increased numbers of NeuroD1 positive cells in the DG, indicating increased levels of adult born neurons in PS19 mice overexpressing noggin (Supplementary Fig S4J).
All in all, noggin overexpression rescued pathologic changes and cognitive decline in the brains of PS19 mice as well as reducing pathology in induced neurons. These findings suggest that BMP inhibition may be a widely applicable therapeutic approach for ameliorating tau-associated neuronal pathologies for tauopathies including both AD and FTD.
Discussion
The fundamental hypothesis underlying the current study is that the age-related increase in BMP signaling in the human brain helps initiate and propagate the pathophysiology of tauopathies. Evidence from both mouse models and iPSC-derived human neurons strongly supports this hypothesis. Further, we found APOE4 and BMP signaling exert synergistic effects on tau phosphorylation and secretion in neurons derived from patients with either familial or sporadic AD genetic backgrounds. APOE4 has been shown to exert a variety of effects that lead to the development of AD pathology.40 However, even early-onset AD takes decades to develop, suggesting that the aging process is involved in triggering the onset of disease. Our observations suggest that the age-related increase in BMP signaling may be one of the triggers that initiate the disease process.
BMP signaling plays an important role in brain, not only in its signature role during neurodevelopment in patterning the nervous system but also in regulating adult neurogenesis throughout life. BMP ligands are highly expressed in the dentate gyrus of the hippocampus, specifically in granule neurons. BMP signals through both the canonical SMAD1/5/8 pathway as well as through non-canonical MAPK-activated protein kinases.36, 41 We found that the effects of BMP signaling on tau phosphorylation are mediated by p38 signaling. Substantial evidence indicates that p38 signaling mediates many of the effects of APOE4 on AD pathology in vivo,22, 42 which helps to explain the synergistic effects of BMP signaling and APOE4 on tau phosphorylation. The APOE gene has a SMAD binding site,43 and BMP signaling increases APOE expression in organs outside of the brain as well as in astrocytes.19 The role of neuronally expressed APOE has been shown in a number of prior studies.23, 44, 45 Nevertheless, the bulk of apoe in the brains of patients with AD is expressed by astrocytes and microglia, and a large body of evidence indicates that glial APOE is involved in the pathophysiology of AD.46-48 Thus, BMP signaling in glia also may interact with APOE in a genotype specific manner to influence neuronal tau phosphorylation detected in our animal model. Future studies that investigate glial-neuron crosstalk in the context of BMP induced AD pathophysiology will further elucidate the role of BMP signaling in AD disease progression.
Independent of glial interactions, we observed that APOE4 iNs express higher levels of TGFβ / BMP signaling-associated genes in comparison to their isogenic APOE3 counterparts, raising the possibility that the APOE4 mutation creates cellular conditions that further increase BMP signaling during the aging process, thereby exacerbating tau pathology. This idea is supported by our noggin treatment experiments in vitro, in which we observed reduced tau phosphorylation in iNs with no exposure to exogenous BMP signaling. APOE4 neurons have been shown to exhibit increased levels of cellular stress, including altered mitochondrial function, lipid metabolism, and proinflammatory factor secretion.45, 49, 50 Elevated BMP signaling may be a part of, or in response to, the stress-related molecular changes in APOE4 iNs. Our findings in vivo that BMP inhibition not only reduces tau-phosphorylation but also improves neuronal survival and synaptic protein expression in PS19 mice suggest that (1) increased BMP signaling activation in the context of tauopathy is detrimental to neuronal health, and (2) the effects of BMP signaling on tau-related pathology are not dependent on APOE4. Our findings suggest a potential generalized role for BMP signaling in 2 different tauopathies, FTD (MAPT P301S mutation) and AD (APOE4 polymorphism). In addition to being a risk factor for AD, APOE4 increases the risk for FTD and lowers the age of onset.10, 11 Thus, our observations about the synergistic effects of BMP signaling and APOE4 may also help to explain the role of APOE4 in FTD as well as AD.
One caveat of this interpretation is the difference in the genetic targets that produce the tau-related pathology in our in vitro (AD/APOE) and the in vivo (MAPT) models. Our study also has some other limitations. First, iPSC-differentiated iNs at 35 days in vitro may not recapitulate all of the aging-associated molecular and cellular properties observed in the brains of patients with. Similarly, our in vitro iN model tests only the acute effects (3DIV) of BMP signaling modulation whereas more chronic treatment of BMP4 or noggin might be necessary to fully model the effects of the age-associated increase in BMP signaling. Second, our in vivo approach for inhibiting BMP signaling used lentiviral expression of noggin that affects multiple cell types at the site of injection, including astrocytes and microglia. Third, we have previously found that noggin overexpression increases neurogenesis and improves cognition in aged wild-type mice. Because we found that noggin treatment of PS19 mice increased neurogenesis, the improvement in cognition in PS19 mice after noggin treatment may reflect, at least in part, the effects on neurogenesis. However, the profound effect of noggin treatment in limiting the pathology in PS19 mice indicates a much broader effect in limiting tau pathology, reducing cell death, and preserving synaptic protein.
Conclusion
In summary, BMP signaling increases levels of p-tau in human iNs, and treatment with the endogenous BMP inhibitor, noggin, greatly reduces levels of p-tau. The effects of BMP signaling on tau phosphorylation are mediated by increased non-canonical p38 kinase signaling. Human iNs that express APOE4 have increased tau phosphorylation compared to isogenic cells that express APOE3, but noggin treatment reduces p-tau in these cells to levels comparable to those in isogenic APOE3 cells. Remarkably, noggin overexpression prevented tau hyperphosphorylation, neuropathological changes, and behavioral abnormalities in mice with the MAPT mutation that causes FTD. Thus, evidence from a mouse model and iPSC-derived human neurons strongly supports the hypothesis that increased BMP signaling is a critical part of the aging process that predisposes individuals to tauopathy pathogenesis. In turn, this raises the possibility that therapeutic interventions aimed at reducing BMP signaling could potentially slow or prevent the development of disease.
Acknowledgments
This research was supported in part by viruses provided by the Northwestern University Skin Biology and Diseases Resource-based Center (P30AR075049), Chicago, Illinois, with support from NIH/NIAMS. The authors would also like to thank the Northwestern University Behavioral Phenotyping Core facility for their assistance. Last, the authors would like to thank the NUseq Core Facility for RNAseq analysis. This work was supported by NIH-MAD training grant T32AG20506 to AA, NIH-NIA training grant F31AG079540 to AKL, and the Davee Foundation.
Author Contributions
A.A. and J.A.K. contributed to the conception and design of the study; A.A., A.K.L., E.T.O., Y.H.T., and C.P. contributed to the acquisition and analysis of data; A.A., A.K.L., C.P., and J.A.K. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Open Research
Data Availability
The datasets generated and/or analyzed during the current study are available in Gene Expression Omnibus repository. Accession number: GSE253647.