Intravenous Thrombolysis 4.5–9 Hours After Stroke Onset: A Cohort Study from the TRISP Collaboration
Abstract
Objective
To investigate the safety and effectiveness of intravenous thrombolysis (IVT) >4.5–9 hours after stroke onset, and the relevance of advanced neuroimaging for patient selection.
Methods
Prospective multicenter cohort study from the ThRombolysis in Ischemic Stroke Patients (TRISP) collaboration. Outcomes were symptomatic intracranial hemorrhage, poor 3-month functional outcome (modified Rankin scale 3–6) and mortality. We compared: (i) IVT >4.5–9 hours versus 0–4.5 hours after stroke onset and (ii) within the >4.5–9 hours group baseline advanced neuroimaging (computed tomography perfusion, magnetic resonance perfusion or magnetic resonance diffusion-weighted imaging fluid-attenuated inversion recovery) versus non-advanced neuroimaging.
Results
Of 15,827 patients, 663 (4.2%) received IVT >4.5–9 hours and 15,164 (95.8%) within 4.5 hours after stroke onset. The main baseline characteristics were evenly distributed between both groups. Time of stroke onset was known in 74.9% of patients treated between >4.5 and 9 hours. Using propensity score weighted binary logistic regression analysis (onset-to-treatment time >4.5–9 hours vs onset-to-treatment time 0–4.5 hours), the probability of symptomatic intracranial hemorrhage (ORadjusted 0.80, 95% CI 0.53–1.17), poor functional outcome (ORadjusted 1.01, 95% CI 0.83–1.22), and mortality (ORadjusted 0.80, 95% CI 0.61–1.04) did not differ significantly between both groups. In patients treated between >4.5 and 9 hours, the use of advanced neuroimaging was associated with a 50% lower mortality compared with non-advanced imaging only (9.9% vs 19.7%; ORadjusted 0.51, 95% CI 0.33–0.79).
Interpretation
This study showed no evidence in difference of symptomatic intracranial hemorrhage, poor outcome, and mortality in selected stroke patients treated with IVT between >4.5 and 9 hours after stroke onset compared with those treated within 4.5 hours. Advanced neuroimaging for patient selection was associated with lower mortality. ANN NEUROL 2023;94:309–320
Introduction
Intravenous thrombolysis (IVT) is the standard reperfusion treatment for patients with acute ischemic stroke up to 4.5 hours after stroke onset.1, 2 Recently, a meta-analysis of three randomized controlled trials (RCT) showed that in selected patients, IVT initiation between >4.5 and 9 hours after stroke onset improved the functional outcome compared with a placebo. Patients were selected with imaging biomarkers showing perfusion mismatch between critically hypoperfused brain tissue and infarct core either on computed tomography (CT) perfusion or on perfusion-diffusion magnetic resonance imaging (MRI) imaging.3 However, this meta-analysis had important limitations, including the small sample size of IVT-treated patients (n = 213) and the high proportion of patients with unknown time of stroke onset (49%), among others. Considering those limitations, experts of the most recent IVT guideline of the European Stroke Organization recommend IVT only for those patients presenting between >4.5 and 9 hours who have both: (1) a known stroke onset and (2) a defined pattern of CT or MR perfusion mismatch. Thus, patients presenting in the >4.5–9 hour time window for whom advanced neuroimaging is not available should not receive IVT. However, the quality of evidence for this recommendation was rated low.2
So far, robust data on IVT in the extended time window outside of RCTs are lacking. Many hospitals treating acute stroke do not have immediate access 24/7 to advanced neuroimaging, and may demand data outside of clinical trials on the benefit of advanced neuroimaging over non-advanced neuroimaging. Furthermore, it is unknown which modality of advanced neuroimaging (CT perfusion, MR perfusion, or MR diffusion-weighted imaging fluid-attenuated inversion recovery [DWI-FLAIR]) should be preferred to select patients for IVT in the extended time window.
By using data from a large prospective IVT registry, we aimed at investigating: (1) if IVT between >4.5 and 9 hours after stroke onset is safe, (2) if the use of advanced neuroimaging is associated with better outcomes and reduced bleeding complications, and (3) if the modality of advanced neuroimaging has an independent impact on outcomes.
Methods
Study Design
For this cohort study, we used prospectively collected data from the ThRombolysis in Ischemic Stroke Patients (TRISP) collaboration, which has been described previously.4 A total of 15 TRISP centers participated in this study. Data collection was carried out locally in each stroke center using a standardized form with predefined variables.5 The anonymized data of the local registries were pooled and analyzed at the stroke center Basel. Variables of interest for the present study were age, sex, National Institutes of Health Stroke Scale (NIHSS) score,6 stroke onset-to-treatment time (OTT), blood pressure before IVT treatment, creatinine and glucose levels, vascular risk factors according to predefined criteria7 and prior treatment with antithrombotic agents (antiplatelet agents or anticoagulants). If the time of symptom onset was unknown (eg, in case of so-called “wake-up strokes”), the time of last seen well was used to calculate OTT. The type of baseline image was retrospectively collected only for patients treated with IVT in the extended time window (OTT >4.5–9 hours). Advanced neuroimaging was defined as CT perfusion, MR perfusion, or MR DWI-FLAIR. In case of performance of MR with DWI-FLAIR and MR perfusion, MR perfusion was used for patient selection. Non-advanced baseline imaging was defined as non-contrast CT and/or CT angiography only.
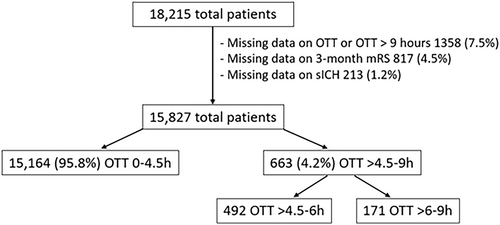
The main outcomes were: (1) the occurrence of symptomatic intracranial hemorrhage (sICH) using the ECASS-II-criteria,8 (2) poor functional outcome at 3 months (defined as a modified Rankin Scale [mRS] score of 3–6. For patients with pre-stroke mRS score >2, poor functional outcome was defined as mRS 4–6), and (3) death within 3 months after stroke. The mRS score at 3 months was assessed by either outpatient visits or telephone calls with patients and/or relatives. Intracranial hemorrhage was monitored by follow-up CT or MRI, as described in prior research.9 Data were collected up to September 2020 (Table 1). All patients with missing data on: (1) OTT, (2) mRS at 3 months, or (3) occurrence of sICH, or with OTT >9 hours and who received endovascular treatment were excluded.
| Center (city, country) | Period | IVT (n) |
|---|---|---|
| Amsterdam, the Netherlands | 01/2000–06/2019 | 908 |
| Basel, Switzerland | 06/1998–09/2020 | 1,577 |
| Belgrade, Serbia | 05/2009–08/2019 | 608 |
| Berlin, Germany | 01/2005–03/2017 | 1,211 |
| Bern, Switzerland | 03/2000–07/2020 | 847 |
| Bologna, Italy | 09/2018–07/2020 | 211 |
| Brescia, Italy | 02/2010–05/2017 | 268 |
| Dijon, France | 03/2007–12/2012 | 388 |
| Heidelberg, Germany | 01/2002–12/2018 | 1899 |
| Helsinki, Finland | 01/2002–12/2017 | 3,342 |
| Jerusalem, Israel | 07/2015–02/2019 | 194 |
| Larissa, Greece | 02/2014–12/2018 | 29 |
| Lausanne, Switzerland | 01/2003–02/2020 | 999 |
| Lille, France | 09/2003–09/2019 | 1,405 |
| Lugano, Switzerland | 01/2014–11/2017 | 101 |
| Modena, Italy | 05/2005–12/2017 | 990 |
| Reggio Emilia, Italy | 01/2015–12/2019 | 514 |
| St. Gallen, Switzerland | 06/2010–08/2013 | 168 |
| Zürich, Switzerland | 01/2014–06/2016 | 168 |
| Total | 15,827 |
Statistical Analysis
Statistical analyses were performed with SPSS Statistics version 25 (IBM Corporation, Armonk, NY, USA) and with R version 4.1.2 (2021-11-01; The R Foundation for Statistical Computing, Vienna, Austria).
We investigated associations between OTT and outcomes using OTT as a categorical variable distinguishing OTT 0–4.5 hours and OTT >4.5–9 hours. OTT 0–4.5 hours served as reference group. Within the OTT >4.5–9 hours (extended time window) subgroup, we investigated associations between the modality of baseline imaging and outcomes by distinguishing advanced versus non-advanced imaging. Non-advanced imaging served as reference group.
Continuous variables were summarized as the median and interquartile range. We used the χ2 test and Fisher's exact test for categorical variables where appropriate, and the Mann–Whitney U test for continuous variables. The association of OTT and imaging modality with poor outcome, death, or sICH was estimated by calculating odds ratios (OR) with 95% confidence intervals (95% CI), using binary logistic regression models and additional propensity score weighting.
To test for differences between the centers, we performed a χ2 test followed by a Bonferroni post-hoc correction.
Because in 2015 acute stroke treatment changed profoundly after introducing endovascular therapy as the gold standard for treatment of acute ischemic stroke with anterior large vessel occlusion, we performed a binary logistic regression analysis using only data of patients with stroke onset starting from January 1, 2015 and later.
As an exploratory analysis, we also investigated the rate of advanced neuroimaging in patients treated with IVT in the extended time window over time (per 5-year intervals).
Binary Logistic Regression Models
For the binary logistic regression models, we allowed the following covariates for OTT 0–4.5 hours versus OTT >4.5–9 hours: age, sex, study center, risk factors (diabetes mellitus, hypertension, coronary artery disease, atrial fibrillation, prior stroke, hypercholesterolemia, smoking), prior anticoagulants, and parameters on admission (NIHSS, systolic blood pressure, creatinine, glucose). The year of treatment was not allowed as an additional covariate, due to correlation with the study centers (see Table 1), but it was analyzed in a sensitivity analysis (stroke onset from 1/1/2015 and later). As data on the use of advanced neuroimaging were only available for the subset of patients treated in the extended time window (OTT 4.5–9 hours), this was also not entered as a possible covariate in the model comparing OTT and outcomes. To avoid overfitting, only covariates reaching a p value of ≤0.1 in a univariate logistic regression analysis were considered for the multivariate binary logistic regression model. The maximum number of potential confounders in the final model was restricted to one-tenth of the number of outcome events. For the binary logistic regression models in the group OTT >4.5 hours advanced versus non-advanced imaging, we used the same covariates as in the model for the main analysis. The covariates used in both final models are listed in the legends of Tables 3 and 5.
Propensity Score Weighting Models
For the propensity score weighted models, the propensity scores for OTT 0–4.5 hours versus OTT 4.5–9 hours and advanced versus non-advanced imaging within the OTT >4.5 hours group (see Tables 3 and 5, respectively) were estimated using binary logistic regression models adjusted for the same covariates as in the corresponding binary logistic regression models (see section above). Then, the average treatment effect was estimated for the main outcomes (poor functional outcome, mortality, sICH) using binary logistic regression models adjusted for the same covariates as in the corresponding binary logistic regression model, weighted with the inverse probability of treatment weights. After balancing with these weights, the maximum standardized mean difference over all models that reached statistical significance was 0.09. The covariates used in the final models are listed in the legends of Tables 3 and 5.
Post-hoc Analyses
We investigated subgroups of advanced neuroimaging (CT perfusion, MR perfusion, MR DWI-FLAIR) versus the non-advanced neuroimaging group regarding poor outcome, mortality, and sICH using binary logistic regression models in patients treated in the extended time window (OTT 4.5–9 hours).
Predictive margins were calculated for each outcome (poor outcome, mortality, sICH) using multivariate generalized linear models using OTT as a continuous variable.
Role of the Funding Source and Ethics
The present study was not funded. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The study was approved by the ethics committee in Basel, Switzerland. The requirement for additional local ethical approval differed between participating centers and was obtained if required. Anonymized data will be shared by request from any qualified investigator.
Results
Data were eligible for analysis for 15,827 (86.7%) of the 18,215 IVT-treated patients. Reasons for exclusion were missing data on OTT or OTT >9 hours (n = 1,358; 7.5%), on 3-month mRS (n = 817; 4.5%), or on sICH (n = 213; 1.2%). Baseline characteristics of the excluded patients are presented in Supplemental Tables S1 and S2. Among eligible patients, OTT was 0–4.5 hours in 15,154 (95.8%) and >4.5–9 hours in 663 (4.2%) patients. Of the 663 patients treated beyond 4.5 hours, 492 (74.2%) patients received IVT within >4.5–6 hours and 171 (25.8%) patients within >6–9 hours (Fig 1).
IVT Treatment between 4.5–9 Hours versus <4.5 Hours after Stroke Onset
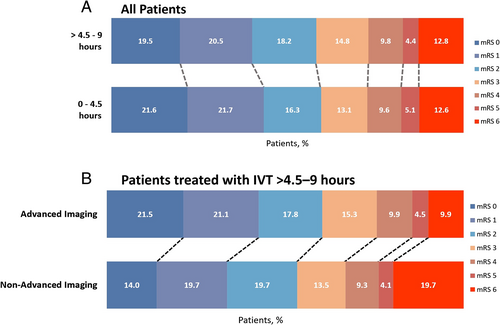
Baseline characteristics are presented in Table 2. Patients with OTT >4.5–9 hours had longer OTT time, were slightly less often independent prior to stroke, had slightly lower creatinine levels, and less often coronary artery disease. All other baseline characteristic (ie, age, NIHSS on admission) did not differ significantly between the two groups. Neither the crude number of outcomes nor the unadjusted or adjusted logistic regression analyses showed any significant differences or association between any time window and outcome (Tables 2 and 3, Fig 2).
| Characteristic | OTT 0–4.5 h | OTT >4.5–9 h | OTT >4.5–6 h | OTT >6–9 h | |
|---|---|---|---|---|---|
| n = 15,164 | n = 663 | p value | n = 492 | n = 171 | |
| Demographics | |||||
| Age, years, median [IQR] | 73 [62–80] | 73 [63–81] | 0.271 | 74 [64–81] | 73 [60–82] |
| Male sex, n (%) | 8,411 (55.5) | 358 (54.0) | 0.452 | 268 (54.5) | 90 (52.6) |
| Independent prior to stroke (pre-mRS 0–2), n (%) | 12,273 (91.8) | 520 (89.3) | 0.032 | 392 (88.9) | 128 (90.8) |
| Stroke characteristics | |||||
| NIHSS, median [IQR] | 8 [5–15] | 8 [4–14] | 0.183 | 7 [4–14] | 8 [5–15] |
| Onset-to-admission time, min, median [IQR] | 142 [105–185] | 314 [287–365] | <0.001 | 298 [284–322] | 415 [388–465] |
| Time of stroke onset unknown/ wake-up stroke, n (%) | NA | 166 (25.1) | NA | 108 (22.0) | 58 (33.9) |
| Medical history | |||||
| Hypertension, n (%) | 10,383 (68.6) | 429 (64.7) | 0.094 | 318 (64.9) | 111 (67.3) |
| Systolic blood pressure, mmHg, median [IQR] | 155 [140–172] | 157 [140–173] | 0.336 | 158 [140–173] | 158 [138–173] |
| Diabetes mellitus, n (%) | 2,903 (19.2) | 123 (18.6) | 0.683 | 90 (18.3) | 33 (19.3) |
| Glucose on admission, mmol/l, median [IQR] | 6.6 [5.7–7.9] | 6.7 [5.7–7.9] | 0.963 | 6.6 [5.7–8.0] | 6.8 [5.6–7.9] |
| Coronary artery disease, n (%) | 2,756 (18.2) | 98 (14.8) | 0.025 | 72 (14.7) | 26 (15.2) |
| Atrial fibrillation, n (%) | 3,478 (25.4) | 145 (23.6) | 0.383 | 115 (25.4) | 30 (19.2) |
| Prior stroke, n (%) | 2,198 (14.5) | 84 (12.7) | 0.182 | 66 (13.4) | 18 (10.5) |
| Hypercholesterolemia, n (%) | 6,781 (44.7) | 278 (41.9) | 0.341 | 199 (40.4) | 79 (49.1) |
| Current (or stopped <2y) smoking, n (%) | 3,017 (21.8) | 142 (22.6) | 0.554 | 107 (22.9) | 35 (22.6) |
| Creatinine on admission, μmol/l, median [IQR] | 81 [68–97] | 78 [65–92] | <0.001 | 78 [66–91] | 76 [63–94] |
| Medication | |||||
| Prior anticoagulation, n (%) | 721 (4.8) | 41 (6.2) | 0.318 | 30 (6.1) | 11 (6.4) |
| Outcomes | |||||
| Symptomatic ICH (ECASS-2 criteria), n (%) | 645 (4.3) | 26 (3.9) | 0.678 | 18 (3.7) | 8 (4.7) |
| Poor functional outcome, n (%) | 6,128 (40.4) | 277 (41.8) | 0.482 | 204 (41.5) | 73 (42.7) |
| Mortality, n (%) | 1915 (12.6) | 85 (12.8) | 0.884 | 62 (12.6) | 23 (13.5) |
- Abbreviation: ICH = intracranial hemorrhage IQR = interquartile range; NA = not available; OTT = onset-to-treatment times.
| Poor functional outcome | Mortality | sICH | |
|---|---|---|---|
OTT >4.5-9 h vs 0–4.5 h |
1.01 (0.83–1.23)1, p = 0.936 | 0.80 (0.60–1.06)1, p = 0.129 | 0.80 (0.52–1.19)2, p = 0.30 |
OTT >4.5–9 h vs 0–4.5 h (symptom onset known) |
1.07 (0.85–1.34)1, p = 0.557 |
0.72 (0.51–1.00)1, p = 0.053 | 0.86 (0.53–1.34)2, p = 0.537 |
OTT >6-9 h vs 0–4.5 h |
0.95 (0.65–1.39)1, p = 0.807 | 0.82 (0.47–1.37)1, p = 0.472 | 0.86 (0.36–1.72)2, p = 0.70 |
| Propensity score weighting | |||
OTT >4.5–9 h vs 0–4.5 h |
1.01 (0.83–1.22)1, p = 0.929 | 0.80 (0.61–1.04)1, p = 0.108 | 0.80 (0.53–1.17)2, p = 0.274 |
OTT >4.5–9 h vs 0–4.5 h (symptom onset known) |
1.07 (0.86–1.33)1, p = 0.546 | 0.71 (0.51–0.98)1, p = 0.042 | 0.86 (0.53–1.31)2, p = 0.514 |
OTT >6-9 h vs 0–4.5 h |
0.95 (0.65–1.39)1, p = 0.8 | NA, no convergence | NA, no convergence |
| Subgroup analysis of patients with stroke onset on 1/1/2015 or later | |||
OTT >4.5–9 h vs 0–4.5 h |
0.92 (0.67–1.26)1, p = 0.608 | 1.18 (0.75–1.81)1, p = 0.453 | 0.74 (0.33–1.43)2, p = 0.415 |
OTT >4.5–9 h vs 0–4.5 h (symptom onset known) |
0.83 (0.57–1.20)1, p = 0.333 | 1.11 (0.64–1.85)1, p = 0.689 | 0.94 (0.39–1.90)2, p = 0.875 |
OTT >6-9 h vs 0–4.5 h |
1.01 (0.57–1.76)1, p = 0.963 | 1.34 (0.55–2.88)1, p = 0.480 | 0.36 (0.02–1.65)2, p = 0.313 |
- Note: Adjusted for: 1age, sex, NIHSS on admission, glucose on admission. 2age, NIHSS on admission. Odds ratio (95% confidence interval), p value.
- Bold value indicates p < 0.05.
- Abbreviation: NA = not available; OTT = onset-to-treatment times; sICH = symptomatic intracranial hemorrhage (ECASS-2 criteria).
Of the 663 patients treated between >4.5 and 9 hours, 497 (74.9%) patients had known symptom onset. When comparing these patients with known symptom onset to the reference group (OTT 0–4.5 hours), the odds for sICH and poor functional outcome remained not significantly different, but there was a signal toward lower mortality for patients treated in the extended time window (ORadjusted 0.80, 95% CI 0.51–1.01; p = 0.053; see Table 2). This difference reached statistical significance when performing propensity score weighting (ORadjusted 0.71, 95% CI 0.51–0.98; p = 0.042; see Table 3).
The results remained unchanged after including year of treatment as an additional covariate in the model (Table S3).
In a sensitivity analysis including patients with stroke onset after January 1, 2015 (n = 5,190), 4,898 patients were treated with OTT 0–4.5 hours (94.4%) and 292 patients with OTT >4.5–9 hours (5.6%). Of the 292 patients treated beyond 4.5 hours, 209 (71.6%) patients received IVT within >4.5–6 hours and 83 (28.4%) patients within >6–9 hours. Neither the crude number of outcomes nor the unadjusted or adjusted logistic regression analyses showed any significant differences or association between any time window and outcome (see Table 3).
IVT-Treatment between >6–9 Hours versus 0–4.5 Hours after Stroke Onset
Baseline characteristics of patients with OTT >6–9 hours compared with those with OTT of 0–4.5 hours were evenly distributed (see Table 2). No significant differences were found between the two groups and any outcome in adjusted regression analyses (see Table 3).
Post-hoc Analysis Using OTT as a Continuous Variable
After adjusting for age, sex, NIHSS on admission, and glucose on admission, the predictive margin models showed an increasing risk for poor outcome, mortality, and sICH with increasing OTT (Fig S1).
Advanced Neuroimaging versus Non-Advanced Neuroimaging in the Extended Time Window
Of the 663 patients treated between >4.5 and 9 hours, 465 (70.1%) had advanced neuroimaging at baseline and 193 (29.1%) did not. In 5 patients (0.08%), the information on the modality of baseline imaging was missing. Patients with advanced neuroimaging were older and more often female, had lower NIHSS on admission, longer OTT time, more often had hypercholesterolemia and unknown stroke onset, and were less likely to have had a prior ischemic stroke (Table 4). The rate of basilar artery occlusion did not differ significantly between patients receiving advanced neuroimaging and patients receiving non-advanced neuroimaging on baseline (3.6 vs 6.3%, p = 0.126). Although the occurrence of sICH and poor functional outcome were similar between both groups, the mortality rate was only half in patients with advanced neuroimaging compared with patients with non-advanced neuroimaging (9.9 vs 19.7%; Table 5, Fig 2). This association remained significant after adjustment for potential confounders in the logistic regression analyses (ORadjusted 0.51, 95% CI 0.28–0.93) and when performing propensity score weighting (ORadjusted 0.51, 95% CI 0.33–0.79; see Table 5).
| Advanced Imaging | Non-advanced Imaging | ||
|---|---|---|---|
| Characteristic | n = 465 | n = 193 | p value |
| Demographics | |||
| Age, years, median [IQR] | 74 [64–81] | 70 [61–80] | 0.052 |
| Male sex, n (%) | 236 (50.8) | 121 (62.7) | 0.005 |
| Independent prior to stroke (pre-mRS 0–2 n (%) | 355 (88.3) | 162 (92.6) | 0.123 |
| Stroke characteristics | |||
| NIHSS, median [IQR] | 7 [4–13] | 8 [5–17] | <0.001 |
| Onset-to-admission time, min, median [IQR] | 320 [290–377] | 300 [282–337] | <0.001 |
| Time of stroke onset unknown/ wake-up stroke, n (%) | 134 (28.9) | 32 (16.6) | 0.001 |
| Occlusion of basilar artery, n (%) | 16 (3.6) | 12 (6.3) | 0.126 |
| Medical history | |||
| Hypertension, n (%) | 299 (65.4) | 127 (65.8) | 0.926 |
| Systolic blood pressure, mmHg, median [IQR] | 156 [140–173] | 159 [139–172] | 0.816 |
| Diabetes mellitus, n (%) | 78 (16.8) | 43 (22.3) | 0.097 |
| Glucose on admission, mmol/l, median [IQR] | 6.5 [5.6–7.8] | 6.8 [5.9–8.2] | 0.062 |
| Coronary artery disease, n (%) | 62 (13.4) | 35 (18.1) | 0.116 |
| Atrial fibrillation, n (%) | 104 (25.0) | 40 (21.3) | 0.320 |
| Prior ischemic stroke, n (%) | 48 (10.3) | 36 (18.7) | 0.004 |
| Hypercholesterolemia, n (%) | 205 (45.7) | 72 (37.3) | 0.050 |
| Current (or stopped <2 y) smoking, n (%) | 95 (21.2) | 47 (27.5) | 0.097 |
| Creatinine on admission, μmol/l, median [IQR] | 78 [66–92] | 78 [62–91] | 0.789 |
| Medication | |||
| Prior anticoagulation, n (%) | 28 (6.0) | 12 (6.3) | 0.627 |
| Outcomes | |||
| Symptomatic ICH (ECASS-2 criteria), n (%) | 19 (4.1) | 7 (3.6) | 0.783 |
| Poor functional outcome, n (%) | 184 (39.6) | 90 (46.6) | 0.094 |
| Mortality, n (%) | 46 (9.9) | 38 (19.7) | <0.001 |
- Abbreviation: ICH = intracranial hemorrhage IQR = interquartile range.
| Poor functional outcome | Mortality | sICH | |
|---|---|---|---|
| Advanced vs non-advanced imaging in onset-to-treatment >4.5–9 h | 0.86 (0.55–1.34)1, p = 0.495 | 0.51 (0.28–0.93)1, p = 0.027 | 1.65 (0.66–4.68)2, p = 0.311 |
| CT perfusion vs non-advanced imaging in onset-to-treatment >4.5–9 h | 0.95 (0.58–1.55)1, p = 0.836 | 0.53 (0.27–1.06)1, p = 0.073 | 1.37 (0.45–4.41)2, p = 0.585 |
| MR DWI-FLAIR vs non-advanced imaging in onset-to-treatment >4.5–9 h | 0.55 (0.24–1.2)1, p = 0.14 | 0.56 (0.2–1.46)1, p = 0.251 | 2.1 (0.51–7.68)2, p = 0.273 |
| MR perfusion vs non-advanced imaging in onset-to-treatment >4.5–9 h | 0.82 (0.44–1.51)1, p = 0.52 | 0.37 (0.13–0.9)1, p = 0.037 | 1.63 (0.4–6)2, p = 0.468 |
| MR (perfusion and DWI-FLAIR) vs CT perfusion in onset-to-treatment >4.5–9 h | 0.78 (0.49–1.241, p = 0.297 | 0.90 (0.42–1.87)1, p = 0.773 | 1.26 (0.46–3.51)2, p = 0.652 |
| Propensity score weighting | |||
| Advanced vs non-advanced imaging in onset-to-treatment >4.5–9 h | 0.87 (0.61–1.24)1, p = 0.434 | 0.51 (0.33–0.79)1, p = 0.003 | 1.81 (0.89–3.98)2, p = 0.121 |
| MR (perfusion and DWI-FLAIR) vs CT perfusion in onset-to-treatment >4.5–9 h | 0.79 (0.56–1.10)1, p = 0.158 | 0.89 (0.52–1.51)1, p = 0.666 | 1.27 (0.62–2.58)2, p = 0.514 |
- Note: Adjusted for: 1age, sex, NIHSS on admission, glucose on admission. 2age, NIHSS on admission.
- Bold value indicates p < 0.05.
- Abbreviation: CT = computed tomography; DWI-DLAIR = diffusion-weighted imaging fluid-attenuated inversion recovery; MR = magnetic resonance; OTT = onset-to-treatment times; sICH = symptomatic intracranial hemorrhage (ECASS-2 criteria).
The results remained unchanged after including year of treatment as additional covariate in the model (Table S4).
Modality of Advanced Neuroimaging in the Extended Time Window
CT perfusion was performed in 251 (54.2%), MR perfusion in 147 (31.6%), and MR DWI-FLAIR in 66 (14.2%) patients. MR perfusion (ORadjusted 0.37, 95% CI 0.13–0.9) was significantly associated with lower mortality compared with the non-advanced neuroimaging group, and CT perfusion (ORadjusted 0.53, 95% CI 0.27–1.06) showed a trend toward lower mortality. The probability of death did not significantly differ between patients with MR DWI-FLAIR and those with non-advanced neuroimaging. No significant association was found between the modality of advanced neuroimaging and poor outcome or sICH (see Table 5).
The results remained unchanged after including year of treatment as an additional covariate in the model (Table S4).
Proportion of Patients Treated in the Extended Time Window and Use of Advanced Neuroimaging over Time
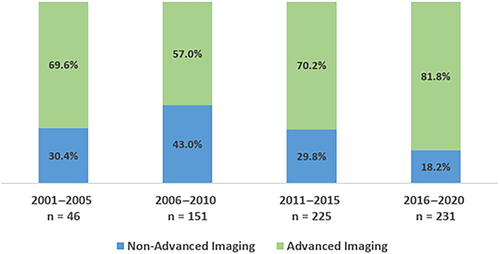
Over the time of this study, both the proportion of IVT treatment in the extended time window, as well as the proportion of patients with baseline advanced neuroimaging, increased in this cohort (Fig 3). When compared with the distribution of cases overall, the ratio for treatment with IVT in the extended time window was the highest during the more recent years (2016–2020; Fig S2).
Discussion
This study comparing outcomes between IVT administration in the standard (0–4.5 hours) and the extended time window (>4.5–9 hours)—with and without advanced neuroimaging—revealed the following key findings: (1) patients treated between >4.5 and 9 hours after stroke onset had similar odds of sICH, poor functional outcome, and mortality compared with those treated between 0 and 4.5 hours; and (2) the use of advanced neuroimaging—especially with MR or CT perfusion based imaging—in the extended time window was associated with lower odds for mortality during the 3-month follow-up, but not with sICH or poor functional outcome.
Recently, the EXTEND trial showed that IVT treatment between >4.5 and 9 hours after stroke onset was effective if patients had a predefined perfusion mismatch on advanced neuroimaging despite higher rates of sICH compared with a placebo.10 This result was confirmed by a meta-analysis of three RCTs (including EXTEND), supporting the importance of advanced neuroimaging for patient selection in IVT >4.5 hours.3 Yet, the total number of patients treated with IVT in this meta-analysis was low (n = 213), and symptom onset was only known for approximately 49% of patients. For patients with stroke on awakening or with unknown onset, the WAKE-UP trial showed that IVT treatment was safe and effective compared with a placebo if a DWI-FLAIR or perfusion mismatch was present on baseline MRI.11, 12
Although the quality of evidence of IVT treatment for patients with stroke on awakening or unknown onset is considered good, it is considered low for IVT used >4.5–9 hours after stroke onset, if the stroke onset is known and if a perfusion mismatch is present on advanced neuroimaging. IVT treatment in the extended time window without advanced neuroimaging is generally not recommended.2 Future RCTs for IVT in the extended time window without endovascular treatment are considered unlikely. Therefore, it is of clinical importance to evaluate the safety of IVT in the extended time window with data from prospective observational studies and to inform clinicians about safety aspects of IVT used >4.5 hours after stroke onset in case advanced neuroimaging is not available.13 As yet, such data are scarce.
One observational study from the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR) compared outcomes in patients treated with IVT between >4.5–6 with >3–4.5 hours and 0–3 hours, respectively.14 In the respective study, only 1.0% of the studied IVT-population (n = 283) was treated between >4.5 and 6 hours after stroke onset, and no information on baseline imaging was provided. No evidence of a difference in regard to sICH, functional outcome, and mortality were found between the three groups. More recently, two retrospective single center studies with smaller sample sizes (n = 274 and n = 53) suggested that patient selection for IVT beyond 4.5 hours with CT perfusion or MR-based imaging was safe, but no comparison with non-advanced neuroimaging or in-between modalities of advanced neuroimaging was performed.15, 16
In the present multicenter study, patients treated in the extended time window had similar outcomes compared with the standard IVT group, indicating that the approach was relatively safe and likely even effective in clinical routine—even for the subgroup of patients treated between >6 and 9 hours after stroke onset. The fact that baseline characteristics were evenly distributed between both groups underlines the robustness of our data. The association between both treatment groups and outcomes remained unchanged in an analysis including patients with stroke onset on 1 January, 2015 and later only. Patients treated in the extended time window with known symptom onset are likely to have even lower mortality compared with those in the standard IVT group.
Interestingly, the percentage of patients receiving IVT with unknown stroke onset or wake-up stroke was much smaller in the present study in comparison with the EXTEND trial (25.1 vs 64.6%).10 This difference is likely explained by differences in treatment decision-making between clinical practice and clinical trials.
The use of advanced neuroimaging (ie, CT perfusion, MR perfusion, or MR DWI-FLAIR) is considered to be the preferred imaging tool for patient selection for IVT in the extended time window.17, 18 Yet, it is unknown whether CT and MRI are two equally valid imaging modalities for patient selection. The results from a recent observational study showed that IVT-treated stroke patients with unknown stroke onset/extended time window had similar probabilities of sICH and favorable functional outcome with multimodal CT (n = 100) compared with multimodal MRI (n = 84).19 However, CT perfusion was associated with significantly shorter door-to-needle time (mean difference −28 minutes). In the present study, the proportion of patients who had multimodal CT or MRI in the extended time window was similar (54 vs 46%). Outcomes did not differ significantly between both groups, suggesting that both imaging modalities are equally valid for patient selection. Of note, the door-to-needle time was also shorter for patients with multimodal CT compared with MRI (57 vs 77 minutes) in our study.
In clinical practice, many hospitals do not have immediate access 24/7 to advanced neuroimaging, and current IVT guidelines do not recommend IVT in the extended time window without the use of advanced neuroimaging.2 A recent observational study compared IVT-treated patients with unknown time of stroke onset and non-contrast CT with matched controls who did not receive IVT. The rate of sICH did not differ significantly between both groups (3.4 vs 0.9%), and IVT-treated patients were more likely to have a decrease of >3 NIHSS points after 24 hours and an excellent functional outcome (mRS 0–1) after 3 months. The authors concluded that patient selection for IVT with non-contrast CT seemed to be safe and possibly effective.20 In the present study, patients with non-advanced neuroimaging had similar odds for sICH and poor functional outcome compared with those with advanced neuroimaging. However, the mortality rate was almost doubled in patients with non-advanced neuroimaging (19.7 vs 9.9%; ORadjusted 0.51, 95% CI 0.28–0.93). This reduction of mortality seemed to be mainly driven by patients receiving perfusion imaging (MR perfusion or CT perfusion). Although the reason for the higher mortality could not be addressed in the present study, it is likely that advanced neuroimaging led to a more careful and precise patient selection. The higher mortality rate was not explained by a different frequency of basilar occlusions (3.6 vs 6.3%, p = 0.126), nor were patients with non-advanced neuroimaging treated more often years back when quality of stroke care in general was lower compared with the more recent years (Fig 3). In addition, we did not find any indicators of ascertainment bias, as missing data for patients with OTT 4.5–9 hours were low in patients with and without advanced neuroimaging (Table S2).
The present results show that the use of advanced neuroimaging is preferable in patient selection for IVT in the extended time window. However, in situations when advanced neuroimaging is not available or feasible, our study may facilitate individual treatment decisions in communication with patients and relatives on IVT treatment in the extended time window without the use of advanced neuroimaging.
Strengths of the present study were: (1) the large sample size (n = 15,827, 663 IVT-treated patients in the extended time window), which allowed adjusting for confounding variables; (2) the high proportion of patients with known stroke onset (75%) in comparison with recent RCTs and smaller observational studies; (3) the comparison of advanced versus non-advanced neuroimaging on baseline; and (4) the comparison between advanced neuroimaging modalities (CT perfusion, MR perfusion, and MR DWI-FLAIR).
The present study had limitations apart from general limitations of observational studies: (1) a risk of selection bias, as we do not know the exact reason why a patient was found to be eligible for IVT in the extended time window or why advanced imaging was applied or withheld;(2) although the overall sample size was large, the proportion of patients treated between >4.5 and 9 hours remained relatively small (4.2%); (3) we were not able to investigate outcomes adjusting for additional imaging criteria (eg, ASPECTS, exact mismatch ratio, core volume); and (iv) data collection was carried out over a long time period, which might lead to a bias due to development of new therapeutic approaches or different selection criteria for patients in the extended time window. Additionally, the end date of data collection of each center was not uniform, possibly resulting in a dysbalance of centers providing more recent data. Yet, adjustment for year of stroke onset did not change the results in the multivariate analyses (Table S3).
Conclusions
In this large dataset from experienced stroke centers, IVT between >4.5 and 9 hours after stroke onset was neither associated with an excess of sICH, worse functional outcome, nor death compared with IVT within 4.5 hours in selected stroke patients. Our data support current guideline recommendations that patients can be selected for IVT even in the extended time window, especially when advanced neuroimaging (CT or MR perfusion) is applied. Further studies on the modality of advanced neuroimaging are warranted.
Acknowledgement
None. Open access funding provided by Universitat Basel.
Author Contributions
V.A., S.T.E., and H.G. contributed to the conception and design of the study. V.A., G.S., L.S.E., C.H., J.F.S., H.H., G.B., D.S., N.M.-M., L.J.S., M.R.H., I.G., D.R.J., Y.B., A.P., R.R.L., G.K., S.W., C.W.C., G.N., G.M.D.M., L.H.B., M.P., P.L., S.R., M.T., A.W., F.C., M.H., H.E., V.P., M.Z., M.A., P.J.N., P.M., A.Z., C.C., C.H.N., P.A.R., S.C., S.T.E., and H.G. contributed to the acquisition and analysis of data. V.A., L.S.E., S.T.E., and H.G. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
The following authors have received speaker honoraria, honoraria for travel, advisory board or consulting fees from Boehringer-Ingelheim, which is producing and selling Actilyse®: D.R.J., Y.B., G.K., S.W., C.W.C., P.L., V.P., A.Z., C.C., C.H.N., P.A.R., and S.T.E. The University Hospital Heidelberg is a sponsor of the ECASS4-trial, examining the role of rtPA in an extended time window, which is financed by Boehringer-Ingelheim. The other authors have nothing to report.