Effect of Mobile Stroke Unit Dispatch in all Patients with Acute Stroke or TIA
Jessica L. Rohmann and Marco Piccininni had equal authorship contribution.
Draft Tweet (179 characters): The benefit of mobile stroke unit dispatch on 3-mo. functional outcomes persists when considering the full spectrum of stroke/TIA patients. @JLRohmann @mpiccininni3 @BerlinStroke.
Abstract
Objective
To determine the effect of additional mobile stroke unit (MSU) dispatch on functional outcomes among the full spectrum of stroke patients, regardless of subtype or potential contraindications to reperfusion therapies.
Methods
We used data from the nonrandomized Berlin-based B_PROUD study (02/2017 to 05/2019), in which MSUs were dispatched based solely on availability, and the linked B-SPATIAL stroke registry. All patients with final stroke or transient ischemic attack (TIA) diagnoses were eligible. The intervention under study was the additional dispatch of an MSU, an emergency physician-staffed ambulance equipped to provide prehospital imaging and thrombolytic treatment, compared to conventional ambulance alone. The primary outcome was the 3-month modified Rankin Scale (mRS) score, and the co-primary outcome was a 3-tiered disability scale. We identified confounders using directed acyclic graphs and obtained adjusted effect estimates using inverse probability of treatment weighting.
Results
MSUs were dispatched to 1,125 patients (mean age: 74 years, 46.5% female), while for 1,141 patients only conventional ambulances were dispatched (75 years, 49.9% female). After confounding adjustment, MSU dispatch was associated with more favorable 3-month mRS scores (common odds ratio [cOR] = 0.82; 95% confidence interval [CI]: 0.71–0.94). No statistically significant association was found with the co-primary outcome (cOR = 0.86; 9% CI: 0.72–1.01) or 7-day mortality (OR = 0.94; 95% CI: 0.59–1.48).
Interpretation
When considering the entire population of stroke/TIA patients, MSU dispatch improved 3-month functional outcomes without evidence of compromised safety. Our results are relevant for decision-makers since stroke subtype and treatment eligibility are unknown at time of dispatch. ANN NEUROL 2023;93:50–63
Introduction
The effectiveness of reperfusion therapies in treating acute ischemic stroke, such as intravenous thrombolysis and mechanical thrombectomy, is highly dependent on the time between symptom onset and treatment initiation.1, 2 Recently, two large, non-randomized, controlled studies and a meta-analysis showed that mobile stroke units (MSUs) considerably reduced onset-to-treatment times in ischemic stroke patients by shifting work-up and treatment into the prehospital setting and were associated with better outcomes at 3-months after stroke.3-5
However, cerebral ischemia patients fulfilling criteria for reperfusion treatments represent only a subset of all patients with stroke symptoms. Indeed, code stroke alarms include numerous cases not qualifying for these treatments, since such indications remain unknown until neurological work-up is performed. Thus, results from studies of ischemic treatment candidates might overlook possible risks for those who cannot benefit from prehospital thrombolytic treatment. Furthermore, selecting only cerebral ischemia patients is prone to selection bias, since some elements of the definition of treatment candidates may, themselves, be affected by MSU dispatch. On the other hand, other aspects of MSU management may help these patients, such as timelier work-up and diagnosis, blood pressure management, anticoagulation reversal, neurological monitoring during transport, and transport to the most appropriate clinical facility.
Therefore, in this extended cohort analysis, we aimed to estimate the effect of additional MSU dispatch among all patients with a final diagnosis of stroke/TIA, which is crucial to inform public health decision-making.
Methods
In the present study, we extended the eligibility criteria of the Berlin-based B_PROUD primary study population to include all stroke/TIA patients, as is detailed in the following sections.
The B_PROUD Study Primary Population
The present study builds on the original B_PROUD study (ClinicalTrials.gov identifier: NCT02869386), an investigator-initiated, nonrandomized, controlled, intervention study that enrolled patients with acute stroke events occurring between February 1, 2017 and May 8, 2019 in Berlin, Germany.4 Comprehensive details have been published elsewhere.4, 6 The objectives of the original study were to study the association between additional MSU dispatch and functional and process outcomes among adult ischemic stroke and TIA patients eligible for reperfusion therapies.4 In total, 1,543 ischemic stroke/TIA patients comprised this primary B_PROUD study population. The B_PROUD study (EA4/109/15) was approved by the Charité – Universitätsmedizin Berlin ethics committee, and an analysis using extended eligibility criteria was pre-specified.6
Intervention
The intervention under study was the additional dispatch of an MSU, while the standard care comparator was conventional ambulance dispatch only. During the study period, 3 MSUs, operating between 7:00 AM-11:00 PM, 7 days per week, were progressively rolled out across Berlin.4 Each MSU was staffed with a radiology technician, an emergency physician with neurology training, and a paramedic and equipped with a computerized tomography scanner for cranial imaging and angiography (CT-A), telemedical access to radiologists, and a point-of-care laboratory. The MSUs could rapidly initiate treatments, including intravenous thrombolysis, blood pressure and pain management, and anti-nausea medication.
Following receipt of an emergency call, an MSU dispatch code was activated at the Berlin Dispatch Center upon suspicion of stroke within 4 hours of onset or unknown onset (based on an emergency interview algorithm). An MSU covering the individual's location, whenever available, was simultaneously dispatched with a conventional care ambulance to the scene; otherwise, only a conventional care ambulance was dispatched. The allocation of MSU dispatch was thus solely dependent on MSU availability, ‘naturally’ creating a control group.
If the conventional care ambulance arrived first, and its personnel concluded that waiting for the MSU was not justified, the MSU could be canceled. Analogous to an intention-to-treat principle, these individuals were still counted in the MSU dispatch group. Berlin emergency medical services (EMS) regulations stipulate transportation of stroke patients to the nearest suitable hospital, i.e., to a hospital with a stroke unit.
The B_PROUD study commenced with one active MSU. In September 2017, a second MSU became active, followed by a third in September 2018, which, after roll-in phases, ultimately achieved an estimated 94% coverage of the Berlin population.4 With the introduction of the third MSU, Global Positioning System tracking was introduced in all vehicles; thereafter, the geographically closest available MSU upon dispatch was sent to the scene.4
Eligibility Criteria for the Extended Cohort Analysis
In the present study, we extended the eligibility criteria of the B_PROUD primary study population to include all stroke/TIA patients, regardless of subtype and any possible contraindications to reperfusion treatments, covered by at least one active MSU, and for whom an MSU dispatch code was activated during operating hours (roll-in phases were ignored).
Patients transported to one of the 15 Berlin hospitals with a stroke unit by either MSU or conventional care ambulance were considered for inclusion in our study. Only patients whose data were available in the linked “Berlin-SPecific Acute Therapy in Ischemic or hAemorrhagic stroke with Long term follow–up” (B-SPATIAL) registry (Clinicaltrials.gov identifier: NCT03027453) could be included in our analyses. The B-SPATIAL registry consists of records from patients aged 18 years or older, with symptom onset within 6 hours of ambulance or hospital arrival, and main hospital discharge diagnosis of acute ischemic stroke (ICD-10: I63 or I64), intracerebral non-traumatic hemorrhage (I61), non-traumatic subdural hemorrhage (I62), or TIA (G45.0-G45.3 or G45.8-G45.9). Patients who had no documented neurological symptoms at the time of EMS arrival as well as no symptoms upon hospital arrival were not documented in the registry. Patients who opted out of data collection were excluded, in accordance with an opt-out follow-up assessment protocol.7 Analyses of B-SPATIAL data were approved by the ethics committee of the Charité - Universitätsmedizin Berlin (EA1/208/21).
Trained and dedicated study nurses collected data, which were stored using the secure, web-based REDCap system,8, 9 monitored by a research associate for completeness and quality.
Outcomes
The primary outcome of our study was functional disability at 3 months measured by the ordinal modified Rankin Scale (mRS) score (range: 0, no symptoms, to 6, death). Since inter-rater reliability of the mRS is improved through the use of structured interviews,10 the B_PROUD study conducted standardized, recorded telephone interviews. Three neurologists blinded to group allocation scored the recorded interviews of patients who were deemed to be part of the primary study population,4 which were edited so that any information that could suggest exposure status was deleted (similar to the PROBE outcome evaluation procedure11).
We computed the median mRS score value out of all available blinded ratings, when available. If the median value fell between 2 discrete mRS categories, then the unblinded rating made by the study nurses was included before taking the median. When patients declined recording of the telephone interviews, when recordings had insufficient sound quality, or when patients were deemed not to belong to the B_PROUD primary study population at the time of the interview, the unblinded ratings were used to determine the mRS score. Individuals who declined a telephone interview were invited to complete a questionnaire sent by postal mail, in which cases, mRS scores were directly determined by the information provided by the patients, without any assistance of the B_PROUD study personnel.
Follow-up mRS information was supplemented by death certificates, which were obtained from the city registration office as a part of regular vital status checks conducted approximately 2 and 4 months after the index event. Patients who died during the 3-month follow-up period received an mRS rating of 6.
During the B_PROUD study period, the co-primary outcome, a 3-tiered disability score at 3 months (mRS 0–3, or if missing, living at home; mRS 4–5 or if missing living in a nursing care facility; mRS = 6), was added as an additional measure of functional outcome because of potential lower-than-anticipated follow-up rates.4
- dichotomous, age-adjusted favorable outcome at 3 months: having mRS 0–1 among patients aged 80 years or younger, or mRS 0–2 among patients aged >80 years;
- alarm-to-imaging time: minutes from dispatch to first cerebral imaging;
- 7-day probability of death;
- proportion of individuals receiving intravenous thrombolysis within 1 hour of dispatch (compared with patients thrombolysed after more than 1 hour or never thrombolysed);
- combined safety outcome for short-term effects: symptomatic secondary intracranial hemorrhage within 36 hours of symptom onset or death from any cause within 7 days.
Other Relevant Variables
In order to identify potential sources of confounding, we created a causal directed acyclic graph using the online application DAGitty12 to identify possible open backdoor paths between the exposure and the outcome based on a priori knowledge about the underlying data generation process (Fig 1).13-19
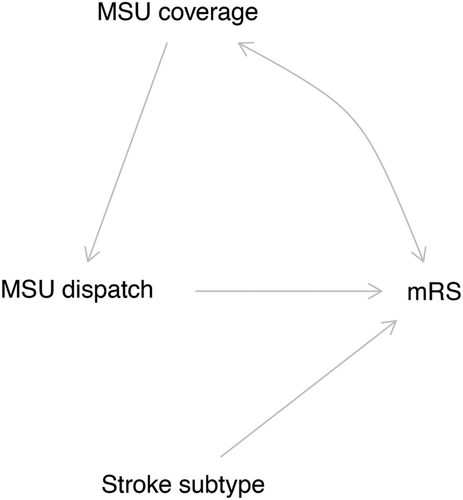
Since the MSU dispatch was solely determined by the MSU availability at the location and index time in this study, the only variable known to influence the exposure and that is possibly associated with the outcome is the “MSU coverage”, as is depicted in our directed acyclic graph (Fig 1). Indeed, the MSU catchment areas had some geographical overlap, meaning that at a certain moment, one location may have been covered by 2 or even 3 MSUs, thereby increasing an individual's likelihood of being allocated to the intervention group. Since the geographical overlap is more common in central areas of Berlin and sociodemographic characteristics and lifestyles are differentially distributed across the city, this variable is also likely associated with the functional outcomes after stroke (indicated by the bidirectional arrow in Fig 1). We operationalized MSU coverage as the number of MSU covering the postal code in which the index stroke event happened, in the quarter of the year in which it occurred. We assumed the catchment areas remained the same after the introduction of the GPS systems into the ambulances.
Another important variable depicted in the directed acyclic graph is stroke subtype (cerebral ischemia vs. hemorrhagic stroke), which is a clear effect modifier for the causal question of interest. The MSU were equipped to determine treatment eligibility and administer effective, evidence-based treatments for ischemic stroke/TIA patients, but not necessarily for hemorrhagic stroke patients. Despite the fact that stroke subtype is an unobservable variable at the moment of MSU dispatch code because it cannot be ascertained until after imaging is performed, the causal effect of the MSU dispatch in these 2 groups has an important clinical relevance. Therefore, we also estimated the average causal effect separately for these 2 groups.
Other collected variables representing relevant ‘traditional’ risk factors for post-stroke outcomes included: age, sex, arterial hypertension (yes/no), diabetes mellitus (yes/no), atrial fibrillation (yes/no), neurological deficits at first assessment (yes/no/no specification possible), independent at home before stroke (yes/no), and prior residence in a nursing care facility (yes/no).
Statistical Analysis
All statistical analyses were performed in accordance with the a priori agreed-upon Statistical Analysis Plan (in Appendix S1). For all variables, we present appropriate descriptive statistics stratified by dispatch group. We tested the conditional independencies implied by the directed acyclic graph using the dagitty R package.20
We estimated the average causal effects of MSU dispatch using inverse probability of treatment weighting (IPTW) with stabilized weights.18 Briefly, this technique uses the probability of MSU dispatch, as estimated from a logistic regression, to reweight the individuals in the study in order to create a pseudo-population with the same size as the original study population in which there is no confounding and the overall proportion of individuals in the intervention group is the same as the one in the original population.18
In accordance with the IPTW approach, we first fit a logistic regression with MSU coverage as an independent numeric variable and MSU dispatch as the dependent variable. We computed the stabilized weights for individuals in the MSU dispatch group (or in the control group) as the ratio of the probability of MSU dispatch (or probability of non-MSU dispatch) as predicted by an intercept-only logistic regression model to the probability of MSU dispatch (or probability of non-MSU dispatch) as predicted by the model containing the MSU coverage as an independent numeric variable. Since stabilized weights generally have higher statistical efficiency than unstabilized weights,18 we opted to use these weights in all of our estimations.
The causal effect of the MSU dispatch on the 3-month mRS was estimated by fitting a proportional odds logistic regression with mRS as the dependent variable and MSU dispatch as the independent variable, applying the stabilized weights. The proportional odds assumption was assessed graphically by plotting the logit of the proportion of the dichotomized mRS variables for each dispatch group.21 We used the same method to estimate the effect of MSU dispatch on the co-primary outcome. The secondary outcomes were analogously analyzed using the same stabilized weights, changing the marginal structural model.
For the alarm-to-image time, we fit a weighted linear regression to estimate the average difference of the variable between the 2 groups in the pseudo-population. For the binary outcomes (age-adjusted favorable outcome, 7-day mortality, intravenous thrombolysis within 60 minutes, and the ‘combined safety outcome’), we fit a logistic regression model with weights as above. Under the assumptions of positivity, conditional exchangeability, consistency, no measurement error, and no model misspecification, the association estimated in these models represent an unbiased estimate of the causal effects of interest.18
In the intracranial hemorrhage subgroup, we observed a major violation of the proportional odds assumption for the primary outcome; therefore, we ran a multinomial regression instead of a proportional odds logistic regression.
Missing values were imputed by Multiple Imputation by Chained Equations with 10 imputed datasets using the R package mice. The set of variables used for missing value imputation included dispatch group, MSU coverage, all outcome variables, and all variables used for adjustment in the sensitivity analysis regression model (described below). Intravenous thrombolysis (yes/no) and alarm-to-thrombolysis time were also used in the imputation process in order to deterministically impute intravenous thrombolysis within 1 hour. The dichotomous age-adjusted favorable outcome was deterministically imputed based on the imputed values of the mRS.
To obtain 95% confidence intervals for the effect estimates, we used Schomaker and Heumann's established BootMI technique.22 For each of 500 bootstrapped datasets drawn from the original dataset containing missing values, 10 datasets were imputed. We ran IPTW and marginal structural models on each imputed dataset and obtained the average regression coefficient estimate across the 10 imputed datasets. Then, the 2.5 and 97.5 percentiles of all obtained average coefficients were used to construct the 95% confidence intervals. Results were considered statistically significant if the 95% confidence interval did not contain the null value.
We additionally performed the above analyses separately among patients with cerebral ischemia only, including the secondary outcomes specific to ischemic stroke/TIA patients. We further present analyses for the primary and co-primary outcomes in hemorrhagic stroke patients only.
Last, for comparability reasons, we performed a sensitivity analysis in which we used the same confounding adjustment approach used in the previously published analysis of the B_PROUD primary study population.4 We fit an ordinal logistic regression with random intercept with the primary (co-primary) outcome as the dependent variable and the previously defined set of “traditional risk factors for post-stroke outcomes” as independent variables. Moreover, another model was also fit including an interaction term for stroke subtype (cerebral ischemia vs hemorrhagic stroke) to estimate the subgroup-specific effects. In order to adjust for heterogeneity, a random intercept was included for each hospital. The point estimate of interest was obtained as the average of the logarithm of the common odds ratio (cOR) estimated from the model in the 10 imputed datasets. Confidence intervals were obtained using BootMI bootstrapping, as above.
All analyses were conducted using R version 4.0.3 and RStudio 2021.09.1.
Results
A total of 16,964 individuals with an MSU dispatch code were assessed for eligibility. Ultimately, 2,266 of 2,397 eligible stroke patients (94.5%) did not opt out and were included (Fig 2). The final study population consisted of 1,125 patients for whom an MSU was dispatched (49.6%) and 1,141 patients for whom only a conventional ambulance was dispatched (50.4%). In the MSU dispatch group, the MSU was canceled after dispatch in 262 (23.3%) cases.
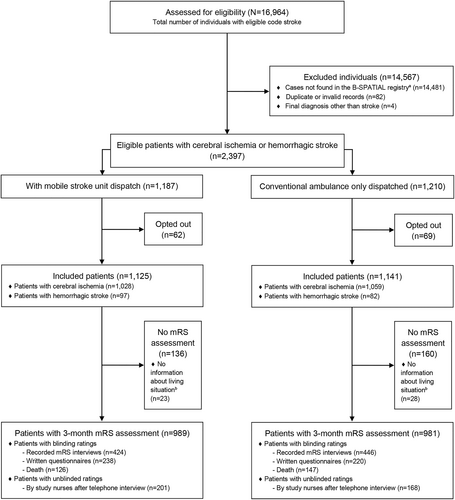
Baseline parameters, short-term outcomes, and process indicators are displayed in Tables 1 and 2. Included patients were 20 to 105 years old; with a mean age of 74 years (SD: 13) in the MSU dispatch group and 75 years (SD: 13) in the group without MSU dispatch; 46.5% and 49.9% were women. Prior to stroke, most patients lived at home without assistance (73.8% vs. 70.3%). The frequency of comorbidities and median National Institutes of Health Stroke Scale (NIHSS) scores upon first assessment were similar between groups. Information regarding neurological deficits upon first EMS assessment was more complete for the MSU dispatch group. The majority of strokes in both groups were ischemic strokes or TIAs (MSU dispatch: 91.4%, non-MSU: 92.8%).
| Parameter | Patients with MSU Dispatch (N = 1,125) | Patients without MSU Dispatch (N = 1,141) |
|---|---|---|
| Baseline demographic and clinical characteristics | ||
| Age | ||
| Mean (SD) | 74 (13) | 75 (13) |
| Median (IQRL) | 76 (66–83) | 77 (68–84) |
| Sex | ||
| Female | 523 (46.5%) | 569 (49.9%) |
| Male | 602 (53.5%) | 572 (50.1%) |
| Living in a nursing care institution | 165 (14.7%) | 185 (16.2%) |
| Living at home without assistance | 830 (73.8%) | 802 (70.3%) |
| Arterial hypertension | 915 (81.3%) | 937 (82.1%) |
| Atrial fibrillation | 361 (32.1%) | 355 (31.1%) |
| Diabetes | 295 (26.2%) | 314 (27.5%) |
| Neurological deficits upon first EMS assessmenta | ||
| Yes | 783 (69.6%) | 533 (46.7%) |
| No specification possible | 288 (25.6%) | 591 (51.8%) |
| No | 54 (4.8%) | 17 (1.5%) |
| NIHSS score upon first assessmentb | nmiss = 9 | nmiss = 37 |
| Median (IQRL) | 4 (2–10) | 4 (2–10) |
| Stroke subtype | ||
| Ischemic | 851 (75.6%) | 868 (76.1%) |
| TIA | 177 (15.7%) | 191 (16.7%) |
| Hemorrhagic | 97 (8.6%) | 82 (7.2%) |
| MSU coverage at site of emergencyc | ||
| 1 MSU | 526 (46.8%) | 788 (69.1%) |
| 2 MSUs | 419 (37.2%) | 291 (25.5%) |
| 3 MSUs | 180 (16.0%) | 62 (5.4%) |
- Abbreviations: EMS, emergency medical services; IQRL, interquartile range limits; MSU, mobile stroke unit; NIHSS, National Institutes of Health Stroke Scale; nmiss, number of missing values; TIA, transient ischemic attack.
- a In the MSU dispatch group, patients were evaluated by MSU EMS staff if the MSU arrived first.
- b The National Institutes of Health Stroke Scale (NIHSS) is a score ranging from 0 to 42, with higher scores indicating greater neurological deficits. The descriptive statistics reported refer to the first NIHSS score available during acute care.
- c MSU coverage varied by geographical location due to partially overlapping catchment areas and subsequent roll-out of the first, 2, and finally 3 vehicles. This variable denotes the number of MSU covering the individual's location (postal code) in the quarter of the calendar year during which the index event occurred.
| Process parametera | Patients with MSU Dispatch (N = 1,125) | Patients without MSU Dispatch (N = 1,141) |
|---|---|---|
| Process parameters | ||
| Symptom onset or LSWb to dispatch | nmiss = 20 | nmiss = 32 |
| Elapsed time in minutes, median (IQRL) | 38 (13–105) | 38 (14–100) |
| Dispatch to first EMS arrival | nmiss = 3 | nmiss = 4 |
| Elapsed time in minutes, median (IQRL) | 9 (7–11) | 9 (7–12) |
| Dispatch to MSU arrival | nmiss = 262 | Not applicable |
| Elapsed time in minutes, median (IQRL) | 16 (13–20) | |
| Dispatch to hospital arrival | nmiss = 1 | nmiss = 2 |
| Elapsed time in minutes, median (IQRL) | 63 (46–79) | 39 (33–45) |
| Among patients receiving thrombolysis | N = 489 | N = 413 |
| Dispatch to needle | nmiss = 4 | nmiss = 4 |
| Elapsed time in minutes, median (IQRL) | 50 (43–65) | 70 (59–85) |
| Among patients receiving mechanical thrombectomy treatment | N = 109 | N = 114 |
| Dispatch to start of mechanical thrombectomy | nmiss = 3 | |
| Elapsed time in minutes, median (IQRL) | 138 (117–167) | 126 (108–155) |
| Symptom onset or LSW to start of mechanical thrombectomy | nmiss = 3 | nmiss = 4 |
| Elapsed time in minutes, median (IQRL) | 171 (140–216) | 158 (127–239) |
- Abbreviations: EMS, emergency medical services; IQRL, interquartile range limits; LSW, last seen well; MSU, mobile stroke unit; nmiss, number of missing values
- a The process parameters were not pre-defined as secondary outcomes and are reported for descriptive purposes.
- b Last-seen-well was assessed and documented by medical personnel after asking the patient (or a relative) at what time the patient had last been observed without acute neurological deficits if symptom onset time was unknown.
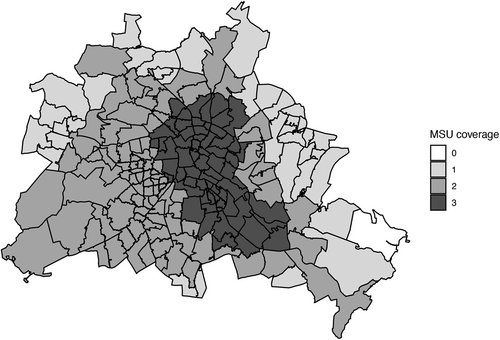
As expected, patients with MSU dispatch were more frequently covered by 3 (MSU: 16.0% vs. non-MSU: 5.4%) or 2 (37.2% vs. 25.5%) vehicles due to the overlapping catchment areas. Fig 3 illustrates the Berlin-wide MSU coverage once the third MSU was introduced.
The median values for the time from symptom onset (or last-seen-well) to dispatch (38 minutes) and from dispatch to first EMS arrival (9 minutes) were the same between groups. The MSU took a median of 16 minutes to arrive at the scene, during which time a conventional ambulance had usually already arrived. In total, 489 (43.5%) patients in the MSU dispatch group received intravenous thrombolytic treatment compared with 413 (36.2%) in the non-MSU group. The median dispatch-to-needle time was shorter in the MSU dispatch group (50 minutes, interquartile range limits [IQRL]: 43–65) compared with the non-MSU group (70 minutes, IQRL: 59–85). A similar proportion of patients underwent mechanical thrombectomy treatment in the 2 groups (MSU: 9.7% vs non-MSU: 10.0%). The median dispatch-to-thrombectomy time was shorter in the non-MSU dispatch group (126 minutes, IQRL: 108–155) compared with the MSU group (138 minutes, IQRL: 117–167).
The conditional independencies implied by our directed acyclic graph were not falsified by any statistically significant associations; as such, no evidence against our directed acyclic graph (Fig 1) was reflected in the data. MSU dispatch was statistically significantly associated with MSU coverage (OR: 2.11, 95% confidence interval [CI]: 1.86 to 2.41), according to the logistic regression model used to estimate the denominator of the stabilized weights.
The primary outcome was assessed in 989 (87.9%) patients in the MSU dispatch group and 981 (86.0%) patients in the non-MSU group. Overall, patients for whom an MSU was dispatched had less global disability as indicated by a favorable shift in the distribution of the mRS score at 3 months (Fig 4 and Table 3). Three-month mRS scores were lower in the MSU dispatch group (unadjusted common OR [cOR]: 0.82, 95% CI: 0.70 to 0.96). The cOR for the co-primary outcome in the unadjusted comparison between groups was 0.91 (95% CI: 0.76 to 1.09).
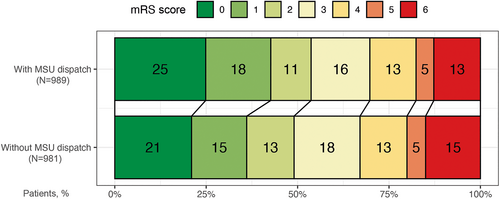
| Outcome variable | Patients with Mobile Stroke Unit Dispatch (N = 1,125) | Patients without Mobile Stroke Unit Dispatch (N = 1,141) | Unadjusted Estimate (95% CI) | Adjusted Estimate (95% CI) |
|---|---|---|---|---|
| mRS scores at 3 months: Primary outcome | Common odds ratio: 0.82 (0.70 to 0.96) | Common odds ratio: 0.82 (0.71 to 0.94) | ||
| Missing | 136 | 160 | ||
| 0 | 246 (24.9%) | 206 (21.0%) | ||
| 1 | 176 (17.8%) | 148 (15.1%) | ||
| 2 | 109 (11.0%) | 127 (12.9%) | ||
| 3 | 159 (16.1%) | 177 (18.0%) | ||
| 4 | 125 (12.6%) | 126 (12.8%) | ||
| 5 | 48 (4.9%) | 50 (5.1%) | ||
| 6 | 126 (12.7%) | 147 (15.0%) | ||
| Tier of disability: Co-primary outcome | Common odds ratio: 0.91 (0.76 to 1.09) | Common odds ratio: 0.86 (0.72 to 1.01) | ||
| Missing | 23 | 28 | ||
| No or mild disability | 779 (70.7%) | 769 (69.1%) | ||
| Severe disability | 197 (17.9%) | 197 (17.7%) | ||
| Death | 126 (11.4%) | 147 (13.2%) | ||
| Age-adjusted favorable outcome at 3 months | Odds ratio: 1.23 (1.03 to 1.48) | Odds ratio: 1.23 (1.04 to 1.48) | ||
| Missing | 136 | 160 | ||
| Favorable outcome | 442 (44.7%) | 388 (39.6%) | ||
| Unfavorable outcome | 547 (55.3%) | 593 (60.4%) | ||
| Time from dispatch to imaging (minutes) | Mean difference: − 3.51 (−8.12 to 1.10) | Mean difference: − 4.18 (−8.64 to 0.79) | ||
| Missing | 50 | 11 | ||
| Mean (SD) | 71 (61) | 74 (49) | ||
| Median (IQRL) | 51 (34–85) | 61 (49–78) | ||
| 7-day mortality | Odds ratio: 1.01 (0.66 to 1.55) | Odds ratio: 0.94 (0.59 to 1.48) | ||
| Alive | 1,081 (96.1%) | 1,097 (96.1%) | ||
| Dead | 44 (3.9%) | 44 (3.9%) |
- Abbreviations: CI, confidence interval; IQRL, interquartile range limits; mRS, modified Rankin Scale; SD, standard deviation.
After multiple imputation and IPTW adjustment, we observed a statistically significant effect of MSU dispatch on the primary outcome, 3-month mRS (adjusted cOR: 0.82, 95% CI: 0.71 to 0.94). Fig 5 provides a visualization of the distribution of the 3-month mRS scores by dispatch group in the pseudo-population. No statistically significant effect was found for the co-primary outcome (adjusted cOR: 0.86, 95% CI: 0.72 to 1.01). The odds of the age-adjusted favorable outcome were 23% higher when MSU was dispatched (adjusted OR: 1.23, 95% CI: 1.04 to 1.48), corresponding to approximately 114 more age-adjusted favorable outcome events in the hypothetical scenario in which all patients had received additional MSU dispatch instead of only conventional care.

Median dispatch-to-imaging times was 51 (IQRL: 34–85) minutes in patients with MSU-dispatch and 61 (IQRL: 49–78) minutes in patients without MSU dispatch. After confounding adjustment, we found no statistically significant difference in mean dispatch-to-imaging time (adjusted mean difference: − 4.18 minutes, 95% CI: −8.64 to 0.79) or 7-day mortality (adjusted OR: 0.94, 95% CI: 0.59 to 1.48). We report all unadjusted and adjusted associations in Table 3.
Patients with Cerebral Ischemia
Among 2,087 patients with cerebral ischemia (TIA or ischemic stroke diagnosis), 1,028 (49.3%) had an MSU dispatch. After adjustment, there was a statistically significant beneficial effect of MSU dispatch on mRS (adjusted cOR: 0.79, 95% CI: 0.65 to 0.92), on the co-primary outcome (adjusted cOR: 0.81, 95% CI: 0.66 to 0.98) and the age-adjusted favorable outcome (adjusted OR: 1.27, 95% CI: 1.07 to 1.54) at 3 months. The odds of receiving intravenous thrombolysis within 60 minutes from dispatch in the MSU group was more than 4 times higher compared with the odds in the conventional care group (adjusted OR: 4.20, 95% CI: 3.37 to 5.35).
Among cerebral ischemia patients, no statistically significant association was found between MSU dispatch and time from dispatch to imaging (adjusted mean difference: − 2.35, 95% CI: −7.99 to 1.88), 7-day mortality (adjusted OR: 0.67, 95% CI: 0.38 to 1.23), or the combined safety outcome (adjusted OR: 0.89, 95% CI: 0.56 to 1.44). The observed number of combined safety outcome events in this group was 90 (4.3%), of which 49 were symptomatic secondary intracranial hemorrhages within 36 hours of symptom onset.
Patients with Intracranial Hemorrhage
In 179 patients with intracranial hemorrhage (spontaneous intracerebral hemorrhage or non-traumatic subdural hematoma), 97 (54.2%) had an MSU dispatch. Among this group, we observed no statistically significant associations between the primary outcome (the OR point estimates for each possible dichotomization of the mRS were 2.95, 1.53, 1.24, 0.92, 0.76, and 0.85; all CIs contained the null value), or the co-primary outcome (adjusted cOR: 0.90, 95% CI: 0.53 to 1.59) and MSU dispatch.
Sensitivity Analysis
In the sensitivity analysis, adjusting for the same variables as used in the published evaluation of the primary study population yielded almost identical results for the primary outcome (adjusted cOR: 0.82, 95% CI: 0.70 to 0.95) and the co-primary outcome (cOR: 0.89, 95% CI: 0.72 to 1.08). Similar results were also obtained among cerebral ischemia patients only (primary outcome, mRS: adjusted cOR: 0.78, 95% CI: 0.66 to 0.91; co-primary outcome: cOR: 0.84, 95% CI: 0.66 to 1.02). As above, no statistically significant association was found among hemorrhagic stroke patients (primary outcome, mRS: cOR: 1.30, 95% CI: 0.68 to 2.41; co-primary outcome: cOR: 1.35, 95% CI: 0.69 to 2.41).
Discussion
Following a small randomized controlled trial, results from only 2 large nonrandomized clinical studies of MSU dispatch (B_PROUD primary population and BEST-MSU) with blinded outcome assessment investigating 90-day mRS have been published;3–5 both studies focused only on patients eligible for reperfusion treatments. The BEST-MSU reported a post-hoc secondary analysis, which found a favorable effect of MSU on mRS at hospital discharge among all patients regardless of treatment eligibility.3 However, this is the first large study that reports the effect of MSU dispatch on 3-month functional outcomes in a cohort of all acute stroke/TIA patients, including those not eligible for reperfusion treatments.
There is clear evidence that MSU dispatch to patients eligible for reperfusion treatments is beneficial among this patient group and this supports the MSU mode of prehospital stroke work-up and care. However, the quantified effect on a larger stroke patient cohort is the one that should ultimately drive policy decision-making because, at the moment of dispatch, individual patient eligibility for reperfusion treatments is unknown.
In this Berlin-based cohort with extended eligibility criteria to include all stroke patients, we observed a beneficial effect of MSU dispatch on 3-month functional outcome as measured by mRS. We did not observe a statistically significant effect for the 3-tiered co-primary outcome. However, the co-primary outcome was introduced following discussion between the Steering Committee and B_PROUD Data and Safety Monitoring Board purely due to concerns with the initially lower-than-anticipated follow-up rates for the primary outcome. It represents a less granular version of the primary outcome, imputed in a more rudimentary way.
Specifically, we found that benefits of MSU dispatch for cerebral ischemia patients persisted, although smaller in magnitude, even when including patients not expected to benefit from MSU management (i.e. those with no disabling neurological deficits at EMS arrival, contraindications against intravenous thrombolysis and mechanical thrombectomy, pre-stroke mobility deficits, recent surgery 4 weeks before the index event, prior stroke within 3 months of the index event, or having an underlying disease with a life expectancy less than 1 year). Among patients with cerebral ischemia, the odds of receiving intravenous thrombolysis within 60 minutes of dispatch in the MSU dispatch group were more than 4 times higher compared with the non-MSU dispatch group; which reflects the fact that MSU dispatch both shortens the time-to-thrombolysis and results in more thrombolysis administration (e.g., makes a higher number of patients eligible for treatment based on time since onset).
Notably, both in the overall cohort and in the subset of patients with cerebral ischemia, we found no evidence of an increased harm by adverse outcomes due to MSU dispatch. We observed no evidence of harm for hemorrhagic stroke patients, who cannot benefit from prehospital reperfusion treatments. This was important to investigate, since this group remains indistinguishable until imaging. More data are needed to quantify any potential benefit of MSU dispatch on functional outcomes among this subgroup.
Perhaps surprising was the smaller difference in dispatch-to-imaging time between groups compared to the difference observed in the primary B_PROUD study population.4 This may be explained by the fact that rapid imaging was no longer a target once contraindications to reperfusion treatments became evident for a subset of the patients. If imaging was then first performed in hospital, the time-to-imaging was longer due to later hospital arrival.
Strengths of our study include the group allocation strategy, which nearly resembles a natural experiment and the fact that we used modern causal inference methods to generate a pseudo-population, which, under a set of commonly relied-upon causal assumptions,18 emulates a randomized controlled trial.23 Variable selection was informed by a directed acyclic graph, a state-of-the-art method that allows for the identification of confounder variables to inform a suitable analytic approach needed to quantify the average (marginal) causal effect.13-19 This stands in contrast to traditional outcome regression-based approaches that deliver “conditional” causal effect estimates, which do not necessarily correspond to the quantity of interest that would have been obtained in an equivalent randomized controlled trial.18
We acknowledge some limitations. First, despite our efforts to include as many individuals with an MSU dispatch code as possible, we could only include patients recorded in the B-SPATIAL registry, which, by definition, did not capture information about those with final non-stroke diagnoses nor those without symptoms upon EMS and hospital arrival. This may have introduced some residual selection bias since it is known that several ambulance dispatches for suspected strokes turn out not to be actual stroke events. Reassuringly, however, in the preceding Berlin-based PHANTOM-S study, approximately half of the 6,182 patients had non-stroke diagnoses and, among these individuals, MSU dispatch was not associated with adverse short-term outcomes prior to hospital discharge.24, 25
Second, although the vast majority of included patients had a blinded outcome assessment, unblinded study nurses ascertained outcomes for patients who were not deceased, did not submit questionnaires, and were not deemed to be in the primary study population upon assessment (with MSU dispatch: n = 201; non-MSU: n = 168).
Third, since this is not a randomized controlled trial, we cannot absolutely rule out possible violations of conditional exchangeability through some unmeasured confounding. For instance, MSU coverage was measured at postal code level, although MSU catchment areas were determined based on travel times, which do not exactly match postal code regions. However, since the sensitivity analysis indicated that our results are robust against different confounding adjustment strategies, we do not anticipate any potential residual confounding would meaningfully change our results.
Finally, our study was conducted in Berlin, Germany, a large metropolitan city with rather short distances from residential areas to hospitals with stroke units and specific EMS protocols. Therefore, our results about the effect of additional MSU dispatch may not necessarily be generalizable to other settings.
Conclusion
Our results suggest that mobile stroke unit dispatch has a beneficial effect when all patients with acute stroke are correctly identified at dispatch. These findings align with the BEST-MSU study results3 and strengthen the recently-published European Stroke Organisation (ESO) guideline recommendations on mobile stroke units for prehospital stroke management.26
Acknowledgements
The authors thank the Berlin Fire Brigade, responsible for the Berlin Dispatch Center and EMS, for their collaboration and support. We are also grateful to the Berlin Data Protection Office (Berliner Beauftragte für Datenschutz und Informationsfreiheit), and specifically to Janet Fahron, data protection officer of the Charité–Universitätsmedizin Berlin, for their advice and consultation. We thank Jakob Beilstein and Fernando Urrutia Gonzalez for their assistance in constructing the MSU coverage variable. We express our continued gratitude to the Berlin Technology Foundation and Berlin Partner for funding previous stages of the Berlin MSU project, neither of whom received compensation for their role in the study. Finally, we thank all collaborating hospitals and the study nurses for their continued support. The B_PROUD study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and the linked B-SPATIAL registry, by the Federal Ministry of Education and Research via the Center for Stroke Research Berlin. M.En. reports funding from DFG under Germany's Excellence Strategy – EXC-2049 – 390688087. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication. Open Access funding enabled and organized by Projekt DEAL.
Author Contributions
J.L.R., M.P., M.Eb., M.En., and H.J.A. contributed to the concept and design of the study; J.L.R., M.P., M.Eb., M.W., J.E.W., E.S., F.G., E.F., P.H., I.L-M., I.R-N., C.H.N, D.G.N., I.S., A.E., M.En., and H.J.A. contributed to the acquisition and analysis of data; J.L.R., M.P., and H.J.A. contributed to drafting the manuscript or preparing the figures.
Potential Conflicts of Interest
C.H.N. reports receiving speaker and/or consultation fees from Boehringer Ingelheim, which produces Alteplase. M.En. reports fees paid to the Charité from Abbot, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Sanofi, Novartis, Pfizer (all related to stroke), all outside the submitted work. H.J.A. reported receiving personal fees from Boehringer Ingelheim, which produces Alteplase and Novo Nordisk, which produces medical products used in the prehospital setting. The other authors have nothing to report. Full disclosures are listed in the submitted ICMJE forms.