Multi-readout thermoplasmonic biosensor for rapid detection of oligonucleotides
Abstract
Developing user-friendly, high-performance sensing devices for nucleic acid detection is crucial for diagnostics, drug development, and personalized therapies. Nanotechnologies offer innovative solutions to meet this need, utilizing the organization and functionalization of nanomaterials. This study presents a paradigm shift in sensing applications, exploiting the morphological and optical properties of gold nanorods (AuNRs) immobilized on a rigid substrate and functionalized with DNA oligonucleotides, creating a DNA–plasmonic microarray. Fabricated through a water-based layer-by-layer electrostatic assembly technique, this method avoids DNA modification. Scanning electron and atomic force microscopy reveal that the AuNRs–DNA microarray has suitable morphological properties to promote DNA hybridization. The photothermal properties of the plasmonic microarray are employed to monitor oligonucleotide hybridization under near-infrared laser irradiation. This approach yields a multi-readout plasmonic biosensor, detecting oligonucleotides by absorption spectroscopy due to its sensitivity to refractive index changes. Additionally, the AuNRs–DNA microarray's unique morphology allows a selective quantification of target DNA sequences through fluorescent imaging and photoluminescence spectroscopy, achieving a detection limit of 0.39 µM, comparable to similar plasmonic-based devices, while adding reusability. This thermoplasmonic-controlled, multi-readout, and reusable biosensor offers promising potential for bioanalytical applications.
1 INTRODUCTION
Nucleic acid detection has become of crucial interest in various biomedical applications, such as environmental monitoring,[1] pathogen detection,[2] drug and vaccine development,[3, 4] disease prevention,[5] diagnostics,[6] and precision medicine,[7] whose personalized diagnoses and therapies represent a turning point in healthcare.[8]
Traditional DNA analysis methods, such as polymerase chain reaction or gel electrophoresis, are laborious and time-consuming. They require expensive equipment, specialized laboratories, and trained personnel and are not suitable for point-of-care use or large-scale distribution.[9, 10]
In contrast, biosensors, especially those based on nanotechnology, are portable, affordable, and easy-to-use sensitive devices that can often provide a rapid and specific response.[11]
This feature marks a significant milestone in point-of-care medicine, as nanotechnology-based biosensors can be engineered to be connected or integrated into portable devices.[12, 13]
Biosensors exploit biological molecules and biochemical reactions to detect other chemical/biological compounds by transducing a given phenomenon caused by recognition into a readable signal.[14]
Biosensors have also been used to detect specific DNA sequences, exploiting the hybridization between two or more complementary DNA strands. This pairing results from hydrogen bonds between nucleic acids, which leads to a highly specific interaction (adenine with thymine and guanine with cytosine).[15] Electrochemical biosensors exploit chemical reactions triggered by hybridization (e.g., redox reactions), which generate a specific electrical signal. In contrast, piezoelectric biosensors rely on the increase in molecular weight caused by the binding of the complementary strand, which alters the piezoelectric oscillations.[10] Optical biosensors are the most commonly used ones for DNA hybridization because they are less expensive and easier to use than the other types. They exploit phenomena such as fluorescence, chemiluminescence, or color changes induced by hybridization. Colorimetric and fluorescent techniques are often combined to enhance the biosensor's sensitivity.[9]
Among optical biosensors, those based on plasmonic nanoparticles (NPs) are particularly advantageous due to the phenomenon of localized surface plasmon resonance (LSPR), described by the Gans theory.[16] Plasmonic NPs are the metallic NPs whose electrons oscillate in groups (named plasmons) when irradiated with visible electromagnetic radiation, and the oscillation leads to the formation of dipoles. For each NP size, the dipoles oscillate at a certain resonance frequency, which is influenced not only by the composition and shape of the NPs but also by the medium in which they are found. For noble metals such as Au, Ag, Cu, and Pt, the resonance frequency lies in the visible range of the electromagnetic spectrum.[17] This effect makes the use of noble metal NPs very interesting because LSPR can be detected by observing intense and characteristic bands in their absorption spectrum.[18]
The resonance frequency depends on the surrounding medium, particularly its refractive index (n), allowing the LSPR to be exploited for biosensing applications. A change in the surrounding medium (e.g., DNA hybridization) produces a variation in n with a consequent change in the resonance frequency proportional to the amount of the analyte.[19]
Among plasmonic NPs, AuNPs are a prime choice due to their stability and biocompatibility.[20] Notably, the first plasmonic biosensor with AuNPs was realized in 1997 to detect DNA hybridization. Appropriately modified AuNPs have been functionalized with a probe sequence, and hybridization with the target has been studied by observing the color change in a dispersion.[21]
In the same years, new techniques for synthesizing non-spherical AuNPs, such as Au nanorods (AuNRs), have begun. These synthesis protocols allow the tuning of AuNR's morphology, thus modulating their optical properties. Indeed, due to their anisotropic shape, the LSPR effect of AuNRs can easily be adjusted to range from the visible spectrum to the near-infrared (NIR) region, as demonstrated by Gans theory.[16] This tunability makes AuNRs particularly attractive for biosensing applications, as they exhibit high sensitivity to changes in the n of their surrounding environment.[22]
AuNRs, due to their anisotropic shape, exhibit two LSPR frequencies, one longitudinal and the other transverse, each associated with the respective absorption band. The transverse plasmon band (TPB) is typically centered around 520 nm in the visible range of the electromagnetic spectrum and is of limited interest for biosensing. In contrast, the longitudinal plasmon band (LPB) is highly sensitive to changes in n, and its position can be tuned from the visible (e.g., 600 nm) to the NIR (e.g., 900 nm) range, according to the aspect ratio (AR) of the AuNRs.[23] As described by the Gans theory, the AR can be tailored to maximize the LSPR's sensitivity to n, optimizing the AuNRs for specific biosensing applications.[22]
Another reason that makes AuNRs particularly interesting is their excellent thermoplasmonic effect, that is, the ability of plasmonic NPs to generate localized heating when irradiated with electromagnetic radiation that matches the LPB frequency.
Specifically, when a light source close to the LPB frequency illuminates AuNRs, the LSPR condition generates a localized temperature increase in the proximity of the AuNR. The absorbed light excites oscillating plasmons, creating a non-equilibrium state. This energy is first dissipated through electron–electron collisions, followed by electron–phonon interactions, and finally through phonon–phonon collisions. The resulting phonon collisions transfer heat to the surrounding medium, facilitating precise thermal manipulation at the nanoscale.[18, 24, 25]
The temperature difference generated is directly proportional to the light source's intensity and the NPs' absorption cross-section. Consequently, anisotropic plasmonic nanostructures, such as AuNRs, which have a higher absorption cross-section compared to isotropic gold nanostructures like gold nanospheres (AuNSs),[26, 27] demonstrate superior light-to-heat conversion efficiency, as reported in previous studies.[24] The thermoplasmonic properties of AuNPs have also been exploited to release DNA for therapeutic reasons[28] and study DNA melting.[29, 30] DNA melting refers to breaking hydrogen bonds between the two double helix strands. The melting temperature is the one at which 50% of the DNA bases will dissociate.[31]
However, the LSPR of AuNRs dispersions has been used for the detection of DNA hybridization in various contexts, for example, for the study, also combined with fluorescence, of human telomere DNA,[32] for the detection of Chlamydia trachomatis using a double probe[30] or to detect a single-base mismatch by exploiting the amplification caused by chain hybridizations.[33] Biosensors with AuNRs and DNA immobilized on a substrate are preferable because they are more practical and stable than biosensors in solution. A significant achievement has been successfully realizing an array of AuNRs on indium tin oxide (ITO) substrates by chromolithography. To functionalize it with the probe strand, it has been necessary to create a monolayer of ω-carboxyalkylthiols, streptavidin and tag the probe with biotin. The resulting biosensor has, therefore, been able to detect the hybridization between the short complementary sequences (CO) through DNA labeling and a complex deposition technique.[34]
A study on the detection of the KRAS gene, which is associated with colon cancer, has been performed using an AuNRs array created by electron beam lithography on ITO. Thiolated biotin, streptavidin, and biotinylated DNA have been used for probe functionalization.[35]
AuNRs have also been arranged vertically by electrochemical deposition. The array has been functionalized with the thiolated probe by covalent bonding and inserted into a microfluidic chamber to study hybridization with fully and partially CO.[36]
However, realizing the mentioned biosensors has required many chemicals, complex techniques, and DNA alteration, which have proved time-consuming and cumbersome. In this work, we take a significant step forward in developing an innovative generation of plasmonic biosensors using a bottom-up approach that enables the fabrication of a controlled AuNR array for multi-readout DNA hybridization detection.
AuNRs have been immobilized on ITO using water-based polyelectrolytes (PEs) via a layer-by-layer assembly. This versatile, customizable, and low-cost electrostatic attraction-based technique eliminates the need for solvents, chemicals, or additional linkers.[37] The resulting AuNR array exhibits excellent optical properties, enabling the immobilization of DNA without altering it and requiring any labeling or tagging, relying solely on electrostatic attraction.
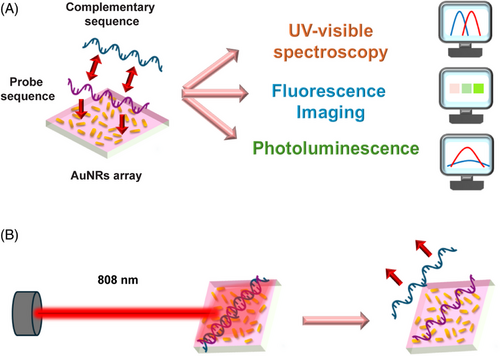
The unique characteristics of the AuNR array facilitate multiple readout methods, including UV–visible spectroscopy, photoluminescence, and fluorescence imaging. These methods allow for a rapid and quantifiable detection of hybridization, reassuring the efficiency of our new approach. Furthermore, when the plasmonic DNA microarray generates photothermal heating, the DNA alters its morphology, causing stretching in the case of the single strand and melting for the double helix. These alterations change the local n of the medium surrounding the AuNRs array. By integrating thermoplasmonics and LSPR phenomena into a single platform, the proposed plasmonic DNA microarray enables the optical monitoring of both the hybridization state and the melting process of DNA. Figure 1 illustrates a schematic of the proposed biosensor and its readout methods.
2 RESULTS AND DISCUSSION
The first step to realize the proposed plasmonic DNA microarray is to obtain an AuNRs array on a transparent substrate. AuNRs dispersed in water were immobilized on an ITO-coated glass substrate using the immersive electrostatic layer-by-layer (eLbL) assembly technique. eLbL assembly is the fabrication of a staking multilayer of polymers held together thanks to electrostatic attractions. This technique, being water-based, is safe and cost-effective, conformal, and easy to scale up. Moreover, it allows tuning the resulting multilayer's optical, morphological, and textural features by suitably modulating the experimental conditions. Finally, it enables the functionalization of a wide range of surfaces, even with complex geometry.[37]
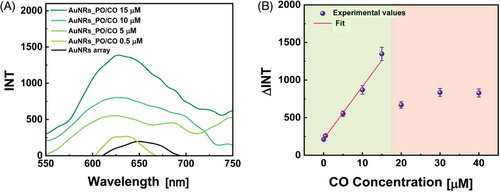
The present work used PEs as suitable polymers to realize a multilayer structure on the ITO substrate suitable for firmly incorporating AuNRs. The layers were assembled on the substrate following the protocol reported in references [38-41]. It promotes the generation of a PEs multilayer (PEM) by alternating depositing cationic and anionic PEs by sequential immersion steps. The PEM was, therefore, assembled due to the electrostatic attractions between two consecutive layers. By using poly(allylamine hydrochloride) (PAH) as a cationic PE and poly(sodium 4-styrenesulfonate) (PSS) as the anionic one, the sequence PAH/PSS/PAH was realized. The AuNRs array was then achieved by immersing the PEM-functionalized substrate in a colloidal dispersion of AuNRs, whose typical absorption spectrum is shown in Figure 2A (dark red curve). The spectrum evidences the typical optical fingerprints of AuNRs. Indeed, the TPB is centered at 512 nm, whereas the LPB shows the maximum plasmonic peak at 791 nm. It is essential to note the value of the TPB's full width half maximum (FWHM), which results in a total of 101 nm. This accounts for the monodispersity and the colloidal stability of the AuNRs dispersion, which reported a Zeta (Z) potential of −43 ± 1.5 mV.[42]
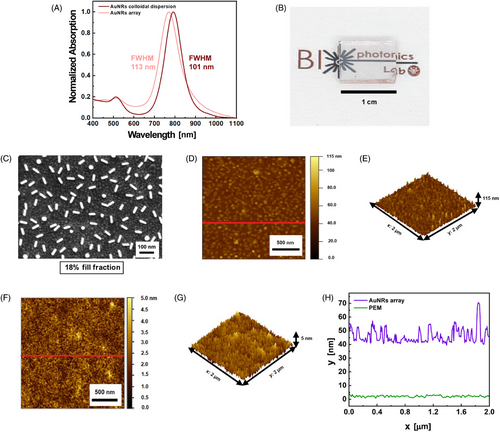
Figure 2B evidences a pinkish color of the realized sample, suggesting a successful deposition of the AuNRs firmly immobilized on the PEM-coated ITO substrate. AuNRs array optical properties are reported in Figure 2A (light pink curve). The latter illustrates the sample's spectroscopic profile, which closely aligns with the colloidal dispersions and highlights the optical fingerprints of AuNRs. Specifically, Figure 2A reveals that for the AuNR array, the TPB is centered at 513 nm, the LPB peaks at 770 nm, and the FWHM is 113 nm, indicating the absence of AuNR aggregates in the AuNRs array. The observed blue shift of the LPB in the AuNRs array is likely due to the decrease inn from water (n = 1.33) to air (n = 1), suggesting the n-sensitivity of AuNRs.[43]
By comparing the two absorption spectra in Figure 2A, it is possible to assess that the utilized immobilization protocol reproduces the optical properties of an AuNRs colloidal dispersion on a transparent substrate in a dry state, as also confirmed by the morphological characterization of the AuNRs array.
The scanning electron microscopy (SEM) micrograph in Figure 2C shows that the AuNRs array consists of AuNRs with an average length of 52 ± 3.0 nm spaced apart on an average distance of 75 ± 20 nm, resulting in a uniform array with a fill fraction of 18% ± 4%.
Figure 2D presents an atomic force microscopy (AFM) image of the AuNRs array, with its three-dimensional topography in Figure 2E. The red line in Figure 2D indicates that the line profile along which the height profile, shown in Figure 2H (purple curve), was measured. Figure 2D,E accounts for the presence of the PEM and AuNRs and reveals that most objects have a height between 45 and 55 nm, a range consistent with AuNRs partially embedded in the PEM, further supporting the role of the PEM-coated ITO substrate in firmly immobilizing the AuNRs into the substrate. Some asperities observed in Figures 2D,E reache heights of up to 100 nm, and a single vertical AuNR does not constitute them, butthe asperities are most likely caused by a cluster of vertically oriented AuNRs. Remarkably, this measurement is not directly connected with the AuNRs length as the magnification and resolution of AFM are not suitable for quantifying the dimensions of the single colloidal nano-object. Indeed, the AFM tip has a finite dimension, making the technique unsuitable for accurately quantifying the dimension of small colloidal NPs. Moreover, due to the convolution effect of the AFM tip, the dimensions of the nanostructures on the image appear larger than the actual NP size. Nevertheless, Figure 2D shows the topographic mapping, the spatial distribution of the AuNRs array that accounts for the presence of the PEM and the AuNRs. The vertically oriented AuNRs appear as spheres in Figure 2C, contributing to the surface roughness, which was measured to be 9.39 ± 0.04 nm using the root mean square (RMS) method and analyzed with Gwyddion software.
The height profile of the AuNRs array is significantly higher and rougher than that of the PEM. As evidenced by the AFM image in Figure 2F, its respective topography in Figure 2G, and the height profile reported in Figure 2H (green curve), the PEM has an average height between 2 and 4 nm, whereas the obtained roughness is 0.54 ± 0.02 nm. The height profile was evaluated along the red line in Figure 2F. Its range and roughness are consistent with previous studies on multilayers formed by PAH and PSS.[44, 45]
The AuNR arrays’ morphology is preserved even after several washing steps in water and ethanol solution (50% in volume) as shown by a dedicated SEM characterization reported in Figure S1. This demonstrates the AuNR array stability under the investigated experimental conditions.
Next, the AuNR arrays were functionalized using oligonucleotide probes (PO), as optical transducers to detect their CO. Figure 3A shows a schematic of the AuNRs_PO realization. Single-stranded DNA (ssDNA) 5′-d(CGA-TGG-GGT-ACG)-3, as the PO, exhibits a weak negative Z-potential (−9.64 ± 0.36 mV) due to negative charges carried by the phosphate groups of the sugar-phosphate backbone of DNA.
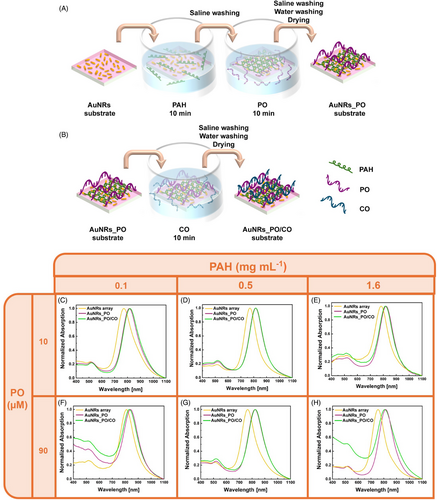
For this reason, the PO was regarded and utilized as a polyanion in the present work.[46, 47]
Indeed, the PO was assembled on the AuNRs array using the PAH as a suitable polycation, resulting in a plasmonic DNA microarray (AuNRs_PO). To investigate the spectroscopic behavior of the AuNRs_PO and optimize its preparation conditions as a function of the PAH and PO concentration, the complementary DNA sequence, 5′-d(CGT -ACC-CCA-TCG)-3′ (CO, 15 µM), was introduced on the plasmonic DNA microarray by adopting physiochemical conditions that promote DNA double-stranded (dsDNA) formation (hybridization), as illustrated in Figure 3B. This process resulted in the formation of AuNRs_PO/CO.
The AuNRs_PO realization was optimized by testing three concentrations of PAH (1.6, 0.5, and 0.1 mg mL−1) and two concentrations of PO (90 and 10 µM).
Figure 3C–H compares the absorption spectra of the AuNRs_PO (purple curves) and AuNRs_PO/CO (green curves), measured as a function of the PAH and PO concentrations. For reference, the absorption spectra of the pristine AuNRs array are also shown (yellow curves).
Figure 3C–H shows that, regardless of the PAH and PO concentrations, all AuNRs_PO samples preserve the spectroscopic profile of AuNRs. This feature indicates that the PO incorporation process does not induce AuNRs aggregation phenomena under the experimental conditions investigated.
Furthermore, in all the experimental conditions studied, the presence of PO determines a redshift of the LPB (Δλ) with respect to bare AuNRs array. Keeping PO concentration constant at 10 µM and increasing the PAH concentration, the measured Δλ, for the AuNRs_PO is 42, 44, and 45 nm for PAH 0.1, 0.5, and 1.6 mg mL−1, respectively (Figure 3C–E). When the PO concentration increases at 90 µM, as reported in Figure 3F–H, the measured Δλ are 45, 57, and 59 nm for PAH 0.1, 0.5, and 1.6 mg mL−1, respectively. Therefore, the Δλ values increase both by increasing the concentration of PAH and PO. These trends indicate the suitability of the eLbL assembly for oligonucleotide incorporation on the AuNRs array, as well as the possibility of controlling their incorporation according to the experimental conditions.
DNA hybridization experiments were performed at this stage using all the AuNRs_PO arrays shown in Figure 3C–H as optical transducers. Leveraging on the AuNRs sensitivity to n changes, absorption spectroscopy was selected as a suitable technique to identify the optimal preparation conditions of AuNRs_PO for CO incorporation. A representative CO concentration of 15 µM was considered and the Δλ between AuNRs_PO and AuNRs_PO/CO was evaluated.
As evidenced in Figure 4A, when the AuNRs_PO was prepared with PAH 0.1 mg mL−1 (Figure 3C), the CO incorporation determined a Δλ of 7 nm using a PO 10 µM, whereas a blue shift of −8 nm was measured when the PO concentration was 90 µM (Figure 3F).
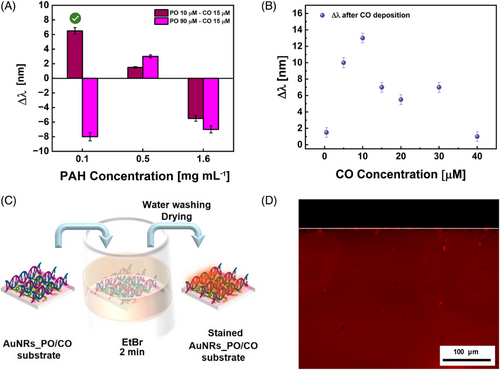
By increasing the PAH at 0.5 mg mL−1, both with PO 10 µM (Figure 3D) and with PO 90 µM (Figure 3G), LPB redshifts after the CO incorporation are 2 and 3 nm, respectively.
Finally, when the CO was hosted on the AuNRs_PO prepared with PAH 1.6 mg mL−1 (Figure 3E,H), the LPB blue shifted of −6 and −7 nm for PO 10 µM and PO 90 µM, respectively (Figure 3E–H and Figure 4A).
These comparisons, summarized in Figure 4A, suggest that CO incorporation is promoted when the AuNRs_PO was prepared using PAH 0.1 mg mL−1 and PO 10 µM under the investigated experimental conditions.
Therefore, the PAH and PO concentrations of 0.1 mg mL−1 and 10 µM were optimal for preparing the AuNRs_PO arrays.
UV–visible absorption spectroscopy validated and quantified the PO incorporation on the optimized AuNRs_PO arrays. This technique enables the quantification of PO solution before and after the AuNRs_PO preparation, thus indicating the PO amount loaded on the AuNRs array.[48] Indeed, UV–visible absorption spectroscopy is a well-established technique for quantifying ssDNA by evaluating the absorbance intensity at 260 nm, as in this region, ssDNA exhibits a typical absorption peak associated with the intrinsic absorption of nitrogen bases.[49]
Hence, as expected, the as-prepared PO concentration resulted in being 35.2 ± 1.0 µg mL−1 (9.9 ± 0.3 µM). After the immersion of the AuNR array, the PO concentration decreased to 29.3 ± 0.8 µg mL−1 (8.2 ± 0.2 µM). This difference suggests that a fraction of PO, approximately at 6 µg mL−1 (2.7 µg), is transferred from the PO solution to the AuNR array. Representative absorption spectra of the 10 µM PO solution, used to estimate the amount of PO on the AuNRs array, are shown in Figure S2. Additionally, in the case of PO 90 µM, the grafted PO quantity is 21.2 µg mL−1 (9.5 µg), and, although it is higher than the one obtained when PO 10 µM was grafted, the CO hybridization did not seem to occur. Moreover, the increase of the PAH concentration should increase the thickness of the PAH layer, thus increasing the distance between the optical transducer (namely, the AuNR array) and the PO/CO duplex. As the distance increases, the intensity of the electric field generated under LSPR conditions exponentially decreases. Consequently, at higher PAH concentrations, the decrease of the electric field causes a reduction in the n sensitivity of the AuNRs array, making the PO/CO hybridization recognition unlikely.
Moreover, a further investigation was performed, reported in Figure S3, and discussed in the SI for the optimized AuNRs_PO array to evaluate the contribution of the PAH layer to the Δλ and further confirm the grafting of PO.
To assess the ability of the AuNRs_PO array to selectively incorporate a CO, the hybridization experiment (as sketched in Figure 3B) was repeated using a 15-µM non-complementary oligonucleotide 5′-d(GCA GAC ATA TCC TAG AGA CAT AT)-3′ (NCO) instead of the CO.
As evidenced in Figure S4, the obtained Δλ (blue-shift) was negligible, suggesting that a DNA sequence not complementary with the one of the PO is not incorporated into the plasmonic DNA microarray. Indeed, the LPB blue-shift can be associated with a decrease in n. This result indicates that the AuNR_PO did not graft the NCO. Accordingly, the optimized experimental conditions for the plasmonic DNA microarray preparation promote only the incorporation of a CO, hindering the grafting of the NCO.
This result indirectly demonstrates that the measured Δλ is ascribable to the hybridization between the PO and CO.
The ability of the AuNRs_PO array to recognize the CO by absorption spectroscopy was also investigated as a function of the CO concentration. Figure 4B reports Δλ values as a function of the CO concentration. As a result, in all the investigated CO concentration ranges, the LPB redshifted. However, it was not possible to identify a defined trend for building a calibration curve suitable for a quantitative analysis.
The presence of CO alters the n experienced by AuNRs_PO, leading to a Δλ that offers qualitative insights. However, the n-sensitivity is insufficient to derive quantitative information.
The UV absorption spectroscopy analyses of the CO solutions, performed before and after the hybridization experiments to evaluate the amount of CO on the AuNRs_PO/CO array, are reported in Figure S5a–f. Experimental results are summarized in the histogram of Figure S5g. It evidences the decrease of the CO concentration in each analyzed solution, during the hybridization experiments. Interestingly, as reported in Figure S5h, by plotting the variation of the CO concentration as a function of the concentration of the CO in the respective reference solution, it is possible to observe a profile compatible with a dose–response curve (logistic fit) in all the investigated ranges, except for the CO concentration 40 µM. This behavior indicates that the CO can be progressively absorbed on the AuNRs_PO/CO array, most likely due to Watson–Crick hydrogen bonds, until reaching a plateau. However, CO incorporation does not occur after a certain concentration threshold. This experimental evidence can be explained by interpreting the DNA as a polyanion-type material. For a defined CO concentration (40 µM in the investigated experimental conditions), the electrostatic repulsions between PO and CO prevail over the Watson–Crick hydrogen bonds, which instead induce the PO/CO hybridization.[50]
Additionally, when the Δλ values are plotted as a function of the CO amount grafted on the substrate, as evidenced in Figure S6, it was not possible to identify a defined trend, in agreement with the results reported in Figure 4B.
To further assess the occurrence of the PO/CO hybridization on the AuNRs_PO array, a representative sample of AuNRs_PO/CO was stained with ethidium bromide (EtBr) (see the sketch in Figure 4C) and analyzed by fluorescence microscopy. The microscopy image in Figure 4D shows a uniform red fluorescence emission. This uniform emission cannot be associated with artifacts, considering the remarkable difference between the AuNRs_PO/CO sample (red area) and its glass substrate border (black area) delimitated by the white line in Figure 4D. The occurrence of red emission can safely be associated with the fluorescence properties of EtBr molecules that intercalate in a dsDNA[51] formed between the PO and the CO on the AuNRs_PO array. Therefore, the uniform red indicates the correct staining technique and a homogeneous distribution of the dsDNA on the substrate.
Interestingly, a different behavior was observed when the hybridization experiment was replicated using the PEM to support the PO/CO hybridization. Experimental data in Figure S5g unequivocally highlight that the PEM_PO incorporates the CO. However, a random trend can be observed by reporting the concentration variation of CO solutions as a function of the respective CO nominal concentration (Figure S5h). Moreover, in a CO concentration ranging from 5 to 30 µM, the CO amount absorbed in the AuNRs_PO array is higher than the CO amount absorbed in the PEM_PO. To address this issue, we compared the plasmonic oligonucleotide microarrays’ topography with the PEM-based microarrays’ topography using the AFM technique.
Figure 5A shows the surface topography of AuNRs_PO, where circular structures, most likely corresponding to ssDNA,[52] result in a surface roughness of 4.10 nm. In contrast, Figure 5B depicts AuNRs_PO/CO, which features more circular clusters, consistent with dsDNA,[53, 54] leading to an increased roughness of 5.63 ± 0.05 nm. These topographies differ markedly from those observed in PEMs. Figure 5C illustrates the surface of PEM_PO, characterized by smaller, more uniformly distributed clusters, resulting in significantly lower roughness, measured at 0.82 ± 0.01 nm. In Figure 5D, representing PEM_PO/CO, the DNA clusters are larger but less densely packed, leading to a roughness of 2.28 ± 0.13 nm and indicating a reduced tendency of PEM_PO to undergo hybridization.
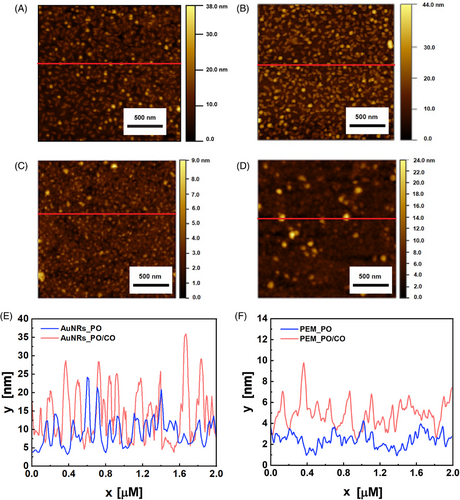
The unique textural properties of the AuNRs array influence the distribution and reactivity of PO.[55] In the plasmonic DNA microarray, the PAH layer, which binds the PO, lies on the AuNRs array. The weak electrostatic attraction between the PAH and the carboxylic group on the AuNRs surface, combined with the distinct surface roughness obtained, results in a loosely packed layer with several binding sites.[56] These characteristics improve the PO distribution and its surface mobility, thus enhancing the hybridization efficiency.[57] In the PEM_PO, instead, the PSS underlies the PAH layer. The interaction between a strong PE (PSS) and a weak PE (PAH) generates a compact bilayer[58] where the PO is densely packed, leading to steric hindrance that limits the hybridization efficiency.[59] Regarding the surface heights, Figure 5E shows the profiles for AuNRs_PO (blue curve) and AuNRs_PO/CO (pink curve). The presence of PO and the underlying PAH layer decreased the average height compared to the bare AuNRs array reported in Figure 2H (purple curve), reaching values between 10 and 15 nm. This is likely attributed to the interaction between PAH and AuNRs, which could have caused a change of orientation of the vertical AuNRs and the disaggregation of any NP clusters. Although the height lowering with adding new layers on the AuNRs may seem counterintuitive, it is a phenomenon reported in the literature that testifies to the complex interactions governing the eLbL.[44, 60] With hybridization, the average height increases, reaching values between 20 and 30 nm, which is compatible with the flexible structure of the double helix, which tends to form bumped structures and loops.[61]
Figure 5F displays the height profiles for PEM_PO (blue curve) and PEM_PO/CO (pink curve), revealing that the heights are significantly lower without AuNRs. When only PO is present, the average height ranges between 3 and 4 nm, slightly higher than that of the bare PEM, as shown in Figure 2H (green curve). Upon the addition of CO, the height increases, reaching values between 5 and 7 nm.
The uniform distribution of dsDNA observed on the AuNRs_PO/CO array and shown in Figure 4D opened up the possibility of analyzing the AuNRs_PO/CO arrays, stained with the EtBr, by an imaging technique based on a laser scanner.
Figure 6A displays the fluorescent scans of the AuNRs_PO/CO at increasing CO concentrations. It can be observed how, for the AuNRs_PO, the fluorescence emission intensity from EtBr is visually lower, whereas it increases for the AuNRs_PO/CO in the CO concentration range from 5 to 15 µM. When the CO concentration rises to 20 µM, the fluorescence decreases. The digital images were elaborated by quantifying the average fluorescence intensity of the pixels in a region of interest (ROI) of 0.75 cm × 0.75 cm for three replicates. The calculated fluorescence intensity, as a function of CO concentration, is shown in Figure 6B. Experimental data show progressive fluorescence intensity increases with the DNA target concentration within a CO concentration domain from 0 to 15 µM. From 15 to 20 µM, the pixel intensity decreases significantly, probably due to self-extinction phenomena associated to the high concentration of the EtBr on the AuNRs_PO/CO surface. A control experiment was performed by scanning stained AuNRs_PO/NCO (NCO 15 µM) and measuring the mean pixel intensity to verify that the fluorescence intensity can be safely associated with the PO/CO hybridization.
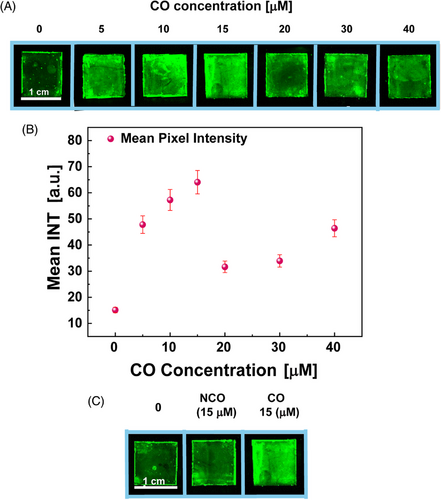
Figure 6C displays the fluorescence in the presence of a non-CO, comparable to the fluorescence of the bare AuNRs array stained with EtBr. Indeed, the analysis of the fluorescence intensities with ImageJ has revealed an intensity of 26.85 ± 1.34 for the bare AuNRs array, whereas the AuNRs_PO/NCO produced an intensity of 33.92 ± 1.70, much lower than the one measured for AuNRs_PO/CO (64.05 ± 3.20), at the same CO concentration. Thus, it confirms that the hybridization that allows the intercalation of EtBr in dsDNA increases the fluorescence emission.
To exploit the AuNRs_PO/CO arrays for quantifying the CO, the arrays were analyzed by photoluminescence (PL) spectroscopy (see Figure S7 for details and Figure S8 for control experiments).
Figure 7A and Figure S9 display representative PL spectra of the AuNRs_PO/CO obtained at different CO concentrations. All the arrays' PL spectra show the typical PL peak at 625 nm associated with the emission of EtBr intercalating in the dsDNA. Conversely, the PL peak at 625 nm is negligible when the AuNRs_PO interacted with an NCO sequence, as confirmed by control experiments in Figure S8, as dsDNA is absent on the plasmonic array. These features indicate successful PO/CO hybridization on the AuNRs array, where the LPB does not quench the emission of the fluorescence reporter under the conditions tested. Notably, when CO concentration is less than 20 µM, the PL intensity progressively increases with the CO concentration, as evidenced in Figure S9a–c and in Figure 7A. When the CO concentration overcomes 20 µM, the PL intensity remains constant at around an average value of 750, in agreement with the pixel intensity values obtained from the digital images. For CO concentrations beyond a specific limit, the emission from the EtBr undergoes self-extinction due to several phenomena, such as the high concentration of intercalating EtBr molecules that remain in the dsDNA on the array after the washing step, the high density of dsDNA that result in a higher EtBr density on the plasmonic DNA microarray, and (for CO concentration 40 µM), the electrostatic repulsions that hinder the CO incorporation.
The R2 coefficient equal to 99 demonstrates the agreement between data and fit. Therefore, the CO concentration range 0–15 µM is optimal as a working range, for using the proposed plasmonic DNA microarray as an oligonucleotide biosensor.
The detection limit was calculated as the ratio between three times the standard deviation of the blank and the slope,[62, 63] obtaining 0.39 µM.
Further control experiments were performed. In particular, PEM_PO/CO with CO between 0 and 40 µM were analyzed with a fluorescence laser scanner and PL in the same ways as AuNRs_PO/CO. As reported in Figure S10a,b, the PL intensity has no trend as the CO concentration varies. Even the fluorescence intensity obtained by processing laser scanning analysis with ImageJ does not show any trend as a function of CO concentration, as reported in Figure S10c,d. This crucial aspect demonstrates the importance of the presence of AuNRs in making DNA quantifiable via PL and fluorescent imaging.
The thermoplasmonic properties of AuNRs can be exploited to induce DNA melting.[24, 29, 30] The melting temperature (Tm) of the PO/CO duplex, influenced by the base composition, GC content, DNA length, and ionic strength, was determined to be 54.1°C in solution under specific conditions (10 mM sodium phosphate, 100 mM NaCl, and 0.1 mM EDTA at pH 7.2) as reported in prior studies.[64, 65] Thermoplasmonic-induced DNA melting was exploited as further evidence of the actual presence of hybridization on the AuNRs_PO/CO. First, the actual photothermal response of the AuNRs array was analyzed. Before illumination, the UV–visible spectrum was acquired to monitor any changes caused by the illumination (black curve in Figure 8A). The AuNRs array was then illuminated for 4 min with an 808 nm NIR laser at a power of 28.3 W cm−2. A thermographic image, acquired when the maximum temperature is reached, is reported in Figure 8B, and it can be noted that the photothermal heating caused 67°C as the maximum temperature. Once cooled, the AuNRs array was again subjected to UV–visible spectroscopy (red curve in Figure 8A), highlighting the total absence of alterations. As reported in the close-up, the LPB did not undergo any shift and remained at 768.5 nm. For the following experiments, the reported illumination conditions were maintained in order to reach temperatures high enough for melting, even though immobilized DNA has lower Tm than DNA in solution.[66] Before illumination, LPB of AuNRs_PO (PO 10 µM) was at 809.5 nm (black curve in Figure 8C), whereas after illumination, it reached 819 nm (red curve in the same figure). This is due to the stretching of the single filament caused by the high temperature.[67] Indeed, by increasing in length and diameter, the PO covers a larger surface on the AuNRs array, causing a change in n that translates into redshift. The maximum temperature reached is 80.1°C, as shown by the thermographic image reported in Figure 8D. Once cooled, the AuNRs_PO was rinsed in Milli-Q water to remove any detached material, and indeed, the subsequent UV–visible spectrum (blue curve in Figure 8C) shows an LPB at 814.5 nm due to the removal of residues. The LPB is still redshifted compared to the position before illumination; therefore, the PO is still present. The various shifts of the LPB are best appreciated in the close-up. For AuNRs_PO/CO, with PO 10 µM and CO 15 µM, the LPB was at 809 nm after PO functionalization (green curve in Figure 8E), whereas with the addition of CO, it reached 813 nm (black curve). After illumination, the LPB did not undergo any shift (red curve), but, following washing with Milli-Q water, a blueshift was recorded, as can be noticed in the close-up. The blueshift brought the LPB back to 808.5 nm (blue curve), practically returning to the condition where only PO was present. This change is due to washing of the detached CO, which confirms the effective initial presence of hybridization on AuNRs_PO/CO. As reported in the thermographic image in Figure 8F, this time the maximum temperature reached was 99.0°C.

The time temperature plots, showing changes relative to room temperature (ΔT), are depicted for the average (Figure 8G) and maximum (Figure 8H) temperatures.
Remarkably, as the initial temperature induces modifications of the reached temperature, for calculating the ΔT values reported in Figures 8G,H, the new initial (room) temperature values were considered for each photothermal characterization experiment. Overall, the average temperature values show a noticeable difference compared to the maximum temperature values. This difference can be explained by noting that the FLIR ResearchIR software considers the maximum temperature to be the temperature of the hottest pixel. In contrast, the average temperature is the mean of all the temperatures measured by the pixels within the ROI used for processing the thermographic images. In parts (g) and (h) of Figure 8, it is evident that the AuNRs array (green curves) heats up less than the AuNRs_PO (blue curves), which in turn is outperformed by the AuNRs_PO/CO (red curves). This behavior is attributed to the fact that the temperature increase due to the thermoplasmonic effect is influenced by the n of the medium surrounding the AuNRs.[24] Indeed, thermoplasmonic experiments, as evidenced in Figure 8B,D,F, revealed that different maximum temperatures were reached after 4 min of laser irradiation, according to the sample under investigation: 67°C for the bare AuNR array, 80.1°C for the AuNR_PO, and 99.0°C for AuNR_PO/CO. At these temperature values, the PEM is still stable,[68, 69] whereas DNA may alter (stretching and/or denaturation). This result highlights that DNA melting occurs primarily due to localized thermoplasmonic heating rather than bulk heating, emphasizing the significance of AuNRs-induced photothermal heating.
In addition, by following the Roper model,[70] the photothermal conversion efficiency (η) of the AuNR array, AuNR_PO, and AuNR_PO/CO were estimated from the time constant values (τ). This parameter was calculated from the cooling phase of the time–temperature plots of Figure 8G,H and is inversely proportional to η. Considering the average temperature plots, the τ values resulted in 27.70 ± 0.31 s for the AuNR array, 25.97 ± 0.40 s for the AuNR_PO, and 30.30 ± 0.83 s for AuNR_PO/CO. When the maximum temperature plots were considered, the τ values resulted in 27.41 ± 0.30 s, 24.94 ± 0.44 s, and 28.70 ± 0.83 s for AuNR array, AuNR_PO, and AuNR_PO/CO, respectively. These results indicate that the investigated samples exhibit a similar η; therefore, the different ΔT values account for their differences in spectroscopic profile and n.
Figure 8H also confirms the maximum temperatures already reported in the thermographic images (Figure 8B,D,F).
These results clearly show that in the presence of nucleotide hybridization, more heat is generated, thus allowing to exploit the thermoplasmonic effect to make the sensor more selective toward fully complementary DNA sequences.[71]
3 CONCLUSION
We have successfully developed a plasmonic-DNA microarray through a scalable, water-based approach based on the eLbL assembly technique. It comprises an AuNRs array assembled on a transparent substrate, functionalized with a PO. Oligonucleotide integration was achieved via a layer-by-layer assembly method, utilizing a cationic PE for anchoring the DNA probe. This approach offers a valuable alternative to traditional techniques such as microfluidics, pin spotting, or covalent bonding for anchoring nucleic acids on surfaces.
AFM analysis and absorption spectroscopy confirmed that the AuNR arrays possess a suitable topography to create a plasmonic bio-interface capable of facilitating oligonucleotide hybridization under optimized conditions. The plasmonic-DNA microarrays demonstrated sensitivity to n, allowing a qualitative detection of DNA hybridization events via absorption spectroscopy. Our study identified the experimental conditions that promote the hybridization of complementary oligonucleotides, offering a clear advantage over simple electrostatic assembly.
Using ethidium bromide as a fluorescent reporter, these microarrays quantified, instead, the complementary DNA strand via two readout techniques: laser scanner imaging, and photoluminescence spectroscopy. Photoluminescence spectroscopy provided a detection limit of 0.39 µM, highlighting the system's sensitivity. From a practical standpoint, the proposed method offers significant advantages. It does not require modifications of the probe DNA strands with thiolated moieties or fluorescent labels, making it more straightforward and cost-effective for practical applications. Additionally, due to the thermoplasmonic properties of AuNRs, photothermal experiments were conducted, inducing DNA melting, which further confirmed nucleotide hybridization and opened the possibility of reusing the microarray while retaining the probe. This work highlights the potential of plasmonic-DNA microarrays in bioanalytical applications.
The proposed DNA plasmonic microarray displays several advantages as a biosensing transducer: (i) it is realized using a safe and water-based, user-friendly, fast, and affordable fabrication method; (ii) the PO does not require modification (e.g., the introduction of thiol group or fluorescent tag is not required); (iii) it displays excellent optical properties; (iv) it enables multiple readout methods (absorption spectroscopy, fluorescence imaging, photoluminescence spectroscopy, and potentially electrochemical methods); (v) it shows a fast response time (the entire process for CO detection requires less than 20 min); (vi) it allows to monitor DNA melting by photothermal heating; and (vii) it can be reutilized exploiting the thermoplasmonic properties.
It paves the way for further advancements in nucleic acid detection technologies and its potential use in various research and diagnostic settings. In this perspective, we foresee the engineering of the proposed system in further technologies, including microfluidic channels and liquid crystalline materials, as well as the possibility to use electrochemical techniques for improving the sensitivity and develop specific algorithms to analyze spectroscopic datasets, enabling the extrapolation of analytically relevant information such as DNA concentration, partial hybridization, or genetic modifications in the target sequence.
4 EXPERIMENTAL SECTION
4.1 MATERIALS
ITO-coated glass slides were purchased from Sigma-Aldrich. Citrate Capped Gold Nanorods (55 nm × 15 nm, AuNRs) were purchased from Nanocomposix, poly(sodium 4-styrenesulfonate) (PSS, Mw = 70,000 Da), poly(allylamine hydrochloride) (PAH, Mw = 50,000 Da), acetone, isopropanol, and methanol were purchased from Merck. Sodium chloride (NaCl M 58.44 g mol−1) was purchased from Sigma-Aldrich and ethidium bromide solution (C21H20BrN3 Emission max 600 nm) was purchased from Fluka Biochemika. Oligonucleotides sequence 5′-d(CGA-TGG-GGT-ACG)-3′ used as PO, its complementary (CO) sequence 5′-d(CGT-ACC-CCA-TCG)-3′ used as oligonucleotide target, and the non-complementary (NCO) sequence 5’-d(GCA GAC ATA TCC TAG AGA CAT AT)-3′ used for control experiments were designed and synthesized by Dr. Annalisa Masi from National Research Council, Institute of Crystallography, and purchased from Aurogene.
4.2 AuNRs array preparation
ITO-coated glass substrates of 1 cm × 1 cm in size were cleaned by sequential sonication for 10 min in methanol and acetone baths, followed by an intermediate rinsing step in isopropanol. After that, the substrates were dried under nitrogen flow and modified with the PEM by assembling the PAH/PSS/PAH sequence.
The PEM was fabricated by sequential immersion of the substrate in PAH, PSS, and PAH for 10 min (1.6 mg mL−1). An intermediate washing step was performed between two consecutive PE solution immersions. Finally, the PEM-functionalized substrate was rinsed in water and dried under nitrogen flow. The incorporation of AuNRs was achieved by immersing the PEM-modified substrate in a water dispersion of AuNRs for 17 h. After the immersion, the resulting AuNRs array was washed with Milli-Q water, dried with a nitrogen stream, and stored in the dark. Washing steps are required as they improve the spectroscopic properties of the AuNRs array, by removing the eventual aggregates and/or impurities, which tend to produce light scattering. The resulting AuNRs array preserves its optical and morphological features if stored in dark conditions, thus ensuring long-term functionality. Moreover, AuNR arrays preserved their morphological stability even after 10 washing steps (10 min) in water and ethanol solution (50% in volume), as demonstrated by a dedicated SEM characterization reported in Figure S1.
4.3 Oligonucleotide synthesis
Oligonucleotides were prepared by automated synthesis using the DMT-and β-(cyanoethyl)phosphoramidite method, on CPG supports (500 Å), with an Expedite 8900 DNA synthesizer (Applied Biosystems) at the 1 µmol scale. Following their synthesis, the DMTr-on ODNs were cleaved from the solid support and deprotected by the method of two syringes using AMA reagent [NH4OH (30%)/CH3NH2 (40%) 1:1] for 10 min at room temperature. The AMA solution containing the cleaved ODN was placed in a sealed vial and heated for 15 min at 55°C. The solvent was then removed in a Speedvac. The crude 5′-DMT-on oligomers were purified and detritylated on-column by RP-HPLC (Grace Vydac C18 column, 5 µm, 50 × 22 mm2). The ODNs were further purified by SAX HPLC (preparative DNA Pac PA-100 column, 5 µm, 22 × 250 mm2). TRIS–HCl 25 mM, pH = 8 (buffer A), and TRIS–HCl 25 mM, NaClO4 0.5 M, pH 8.0 (buffer B) were used at a flow rate of 9 mL min−1 eluting with 2%–30% B in 30 mins, 30% B for 10 min, then 30%–45% B in 5 min monitoring at 254 nm. The purified fractions were concentrated, desalted on Water SepPak-C18-cartridges and lyophilized again. The final DNA yield was estimated by UV absorption in aqueous solution measured at 254 nm on a Cary 100 UV–visible spectrometer following standard procedures.
MALDI-TOF mass spectrometry and analytical SAX HPLC chromatography were used to characterize the purified ODNs.[65, 72] The synthesis occurred at the Institute of Crystallography of the National Research Council.
4.4 AuNRs array functionalization with oligonucleotides
All oligonucleotide solutions (PO, CO, and NCO) were diluted in NaCl 0.1 M to reach the desired concentrations. AuNR arrays were immersed in a PAH solution for 10 min, washed with saline solution, immersed in PO for 10 min, and washed again first with saline and then with Milli-Q water. The obtained AuNRs_PO substrates were then dried under a nitrogen stream and characterized. They were subsequently immersed in CO or NCO for 10 min, washed with saline solution and Milli-Q, and dried under a nitrogen stream, obtaining the AuNRs_PO/CO or AuNRs_PO/NCO substrates, respectively. The optical properties of the AuNRs_PO/CO and AuNRs_PO/NCO substrates are maintained unaltered for 48 h when stored at 4°C.
4.5 UV–visible spectroscopy
UV–visible absorption spectroscopy was used to measure the optical density (OD) of colloidal dispersion of AuNRs, for the optical characterization of AuNRs array, to assess the correct functionalization with PO, to verify the presence of hybridization event and to monitor the effects of photothermal experiments. Measurements were performed using a Lambda 365 spectrophotometer from PerkinElmer. The spectra are normalized to the LPB absorption intensity for better visualization of the spectral shift caused by the change in n. UV spectroscopy was also used to analyze oligonucleotide solutions as a control experiment to evaluate their effective adsorption onto the substrates. A Varioskan LUX multimode microplate reader equipped with a µdrop plate was employed for these control experiments.
4.6 SEM and AFM characterization
To investigate the morphology of the AuNR arrays, SEM micrographs were obtained using a high-resolution scanning electron microscope (AURIGA from Zeiss). Measurements were performed at the interdepartmental research center on nanotechnologies applied to engineering (C.N.I.S.—Sapienza University of Rome).
AFM was used to investigate the surface profile of AuNR arrays, AuNRs_PO, AuNRs_PO/CO, PEM, PEM_CO, and PEM_PO/CO substrates. Measurements were performed at C.N.I.S.—Sapienza University of Rome using the AFM Veeco Icon and the AFM Veeco Multimode, both in tapping mode.
4.7 Dynamic light-scattering measurements
To measure the Z-potential of PO and PAH, the Zetasizer Pro from Malvern Panalytical was used in electrophoretic light scattering mode. The Z-potential was also measured for an AuNRs dispersion 0.01 mg mL−1 and OD 5 (pH 5, Milli-Q water). The instrument exploited a 633 nm He–Ne laser with a power of 10 mW. Measurements took place at the Institute of Crystallography of the National Research Council.
4.8 Photoluminescence
To verify the actual presence of DNA, the presence or absence of hybridization, and to quantify the DNA using a calibration curve, the substrates were analyzed with photoluminescence (PL), after being stained with the intercalating fluorophore ethidium bromide (EtBr). An EtBr solution in a concentration of 0.71% was deposited on the substrates. After 2 min, a washing step was performed, and the process was repeated after another 2 min. The substrates were allowed to dry under a chemical hood. The PL spectra of stained substrates were obtained using a FL8500 PL spectrophotometer from PerkinElmer.
4.9 Fluorescence imaging
Stained substrates were analyzed with two fluorescence imaging techniques. AuNRs_PO/CO substrates were observed with fluorescence microscopy to verify the presence and uniformity of hybridization. The images were collected using a ZEISS Axiolab 5 fluorescence microscope. To obtain further proof of hybridization and an additional readout method, a Typhoon FLA 9000 laser fluorescence imaging scanner was employed. EtBr-stained substrates were analyzed with the 532 nm laser, as recommended by the manufacturer.
4.10 Photothermal experiments
Photothermal experiments were performed to induce DNA melting to confirm further that the observed phenomena are due to hybridization. AuNRs array, AuNRs_PO, and AuNRs_PO/CO were illuminated with an 808 nm continuous NIR laser (PowerLine from Coherent). The NIR laser beam has a rectangular profile, so it was necessary to convert it to a quasi-circular one using a 20 cm focal length elliptical lens. Illumination was performed at a power density of 28.3 W cm−2 for 4 min, and then the samples were allowed to cool down for 2 min. The temperature changes were recorded using an FLIR A655sc thermal camera, whose resolution is 640 × 480 pixels. The accuracy on temperature is ±0.2°C, and the recording started 5 s before the laser was turned on to obtain the baseline. The camera also evaluated the spatial heating distribution on the sample surface in a defined ROI. The acquired images were processed using FLIR ResearchIR software. After the experiments, AuNRs_PO and AuNRs_PO/CO were rinsed with Milli-Q water to remove any detached DNA.
4.11 Statistical analysis
UV–visible absorption spectra were normalized between 0 and 1 using OriginLab software. This software was also used to analyze the statistical analysis of data from spectroscopy, photoluminescence, fluorescence imaging, and photothermal measurements. The data in Figures 4, 6, and 7B are reported as mean values ± standard deviation of three replicates. The goodness of the linear fit was confirmed by calculating the R2 coefficient, whereas the χ2 test was performed for the logistic fit. ImageJ software was employed for the statistical analysis of SEM micrographs, whereas the Gwyddion software was used for AFM micrographs. For each type of substrate, the error in the roughness was estimated from the standard deviation obtained from the analysis of five different AFM micrographs at the same magnification. The error of the fill fraction was calculated from the standard deviation obtained from the analysis of five different SEM images at the same magnification.
ACKNOWLEDGMENTS
This work has been supported by EU funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, CUP B53C20040570005 INF-ACT), by the “NATO—Science for Peace and Security Programme (SPS-G7425, CLC-BIODETECT)”, and by the Air Force Office of Scientific Research, Air Force Material Command, US Air Force. “Digital optical network encryption with liquid-crystal grating metasurface perfect absorbers” FA8655-22-1-7007 (P. I., L. De Sio, EOARD 2022–2025).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.