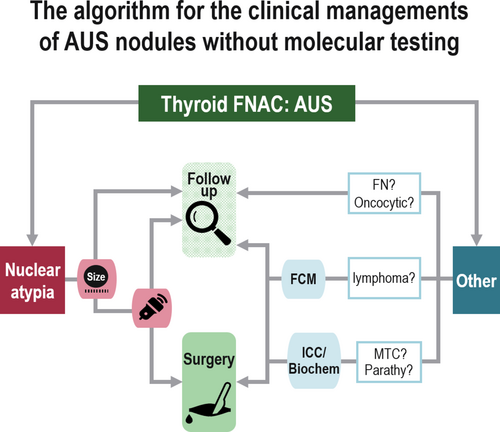
Proposal for Clinical Management of Nodules Diagnosed as Atypia of Undetermined Significance via Thyroid Fine-Needle Aspiration Cytology in the Absence of Molecular Testing
Funding: The authors received no specific funding for this work.
Mina Desai Early Career Investigator Award: We nominate Ayana Suzuki.
ABSTRACT
Objective
Molecular testing is recommended for risk stratification of atypia of undetermined significance (AUS) nodules in the USA; however, it is not routinely performed in some countries owing to limited availability and affordability. Here, we propose a risk stratification algorithm for AUS nodules when molecular testing is unavailable.
Methods
We examined 304 (4.3%) AUS nodules among 7073 thyroid fine-needle aspiration cytology specimens examined at Kuma Hospital from January 2020 to December 2020. Clinical data were obtained from the medical records of Kuma Hospital.
Results
AUS with nuclear atypia and AUS-other each accounted for half of the total AUS nodules. The repeat aspiration rate was 19.7%; 61.7% of the nodules were reclassified as benign or malignant upon repeat aspiration. Resection rate and overall risk of malignancy (ROM) were 32.6% and 12.8%, respectively. Architectural atypia showed the lowest (1.1%) overall ROM in the AUS nodules. For AUS with nuclear atypia, nodules ≤ 10 mm in size showed significantly lower overall ROM than those of > 10 mm, and nodules with ultrasonographically low suspicion showed significantly lower overall ROM than those with intermediate to high suspicion. AUS nodules with atypical lymphoid cells, possible medullary thyroid carcinoma, or possible parathyroid lesion were confirmed using flow cytometry, biochemical testing using needle washout fluid or immunocytochemistry, respectively.
Conclusions
Our proposed clinical management algorithm for each subdivision according to cytological findings, based on repeat aspiration rates, ROM, ultrasound findings and results of ancillary tests except for molecular testing, should be useful for the clinical management of AUS nodules.
Graphical Abstract
1 Introduction
The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was proposed for standardising the reporting of thyroid fine-needle aspiration cytology (FNAC) specimens in 2007 and describes the guidelines for clinical management and risk of malignancy (ROM) for each diagnostic category of thyroid nodules [1]. TBSRTC was revised twice, in 2017 and 2023 [2, 3]. The category ‘atypia of undetermined significance’ (AUS) is reserved for specimens with less atypia, nuclear and/or other in nature, which is insufficient to be classified as ‘follicular neoplasm (FN)’, ‘suspicious for malignancy’ or ‘malignant’ [3]. Because of heterogeneous lesions, with widely varying resection rates [4-6], calculating the ROM has been challenging for this category. The third edition of TBSRTC was updated to subclassify AUS into ‘AUS with nuclear atypia (AUS-NA)’ and ‘AUS-other’ based on differences in ROMs—the former is associated with a low level of concern for papillary thyroid carcinoma (PTC) or noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) and the latter has non-nuclear features in an AUS interpretation. The clinical management of AUS involves repeat FNAC, molecular testing, diagnostic lobectomy or surveillance, irrespective of the subclassification [3].
AUS nodules are resected when they exhibit worrisome clinical or ultrasonographic features, an abnormal repeat aspiration result and/or an abnormal result in molecular testing [7]. Although molecular testing is useful in stratifying cytologically indeterminate thyroid nodules into clinically meaningful risk categories [8], it is limited by expensive gene panel testing, which is not available worldwide [6, 9]. It is, therefore, imperative to establish management protocols for AUS nodules that can minimise unnecessary surgical interventions, even in the absence of molecular testing. In this study, we aimed to develop a risk stratification algorithm for AUS nodules when molecular testing is not possible. The findings in this study will provide a reference for institutions where molecular testing is not available.
2 Materials and Methods
2.1 Patients and Clinical Investigation
The study protocol was reviewed and approved by the Institutional Review Board of Kuma Hospital. FNAC was performed for 7073 thyroid nodules at Kuma Hospital, Japan, from January 2020 to December 2020. All subjects provided informed consent. Among them, 304 nodules (4.3%) originally categorised as AUS were included in this study. Clinical data were obtained from the medical records of Kuma Hospital. Diagnostic criteria for AUS nodules were based on the third edition of TBSRTC [3]. AUS nodules were subclassified into AUS-NA and AUS-other. The former was further subdivided into specimens with focal nuclear atypia, extensive but mild nuclear atypia, atypical cyst-lining cells, histiocytoid cells and nuclear and architectural atypia; the latter was subdivided into architectural atypia, oncocytic atypia, atypia not otherwise specified and atypical lymphoid cells. Preoperative molecular testing using aspirated materials was not performed in any of the cases. The follow-up period after the initial FNAC ranged from 3.3 to 4.2 years (mean 3.7 years).
2.2 Ultrasonographic Characteristics and Classification
Tumour size was determined using ultrasonography and defined as the maximum diameter of the tumour recorded at the time of FNAC. The findings were classified into five categories: benign, very low suspicion, low suspicion, intermediate suspicion and high suspicion, based on the American Thyroid Association guidelines [10]. Of these, benign and very low suspicion were not included in this cohort.
2.3 Fine-Needle Aspiration Cytology
FNAC was performed according to the guidelines of The Japan Association of Breast and Thyroid Sonology [11], and nodules ≥ 5 mm in size and suspected to be malignant on ultrasonography were indicated. FNAC specimens were prepared using the press and release method [12] and smeared with the Papanicolaou stain.
2.4 Ancillary Tests Using Aspirated Materials
Calcitonin measurement using needle washout fluid was performed for a case of possible medullary thyroid carcinoma. After smearing, the aspirate remaining in the needle was rinsed with 0.5 mL normal saline and analysed for calcitonin using the Elecsys Calcitonin test system (Roche Diagnostics, Tokyo, Japan). Calcitonin level > 21.0 pg/mL was considered positive [13]. Flow cytometry was performed for cases of possible lymphoma. Aspirates were placed in a cell preservation liquid (5 mL, H00; Nissui Pharmaceutical, Tokyo, Japan) and analysed using a FACSCanto II Cell Analyser (BD Biosciences, San Jose, CA, USA). A κ/λ ratio > 3 or < 0.5 was defined as light-chain restriction [14]. Both calcitonin measurement and flow cytometry were outsourced (SRL Co., Tokyo, Japan). GATA-3 immunocytochemistry was performed for a case of possible parathyroid adenoma. Immunostaining was performed using an anti-GATA-3 antibody (L50-823, 1:400, Biocare Medical, Concord, CA, USA) employing an automated Leica Bond-Max system and Bond Refine detection kit (Leica Microsystems, Wetzlar, Germany) in accordance with the manufacturer's instructions. Strong nuclear positivity was defined as positive [15].
2.5 Risk of Malignancy
Overall and resected ROMs were defined as percentages of malignant nodules in all and resected AUS nodules, respectively.
2.6 Statistical Analyses
Statistical analyses were performed using the Stat Flex v.6 statistical software (Artech Co. Ltd., Osaka, Japan). A p-value < 0.05 was considered statistically significant in Fisher's probability test.
3 Results
Table 1 shows the original cytological interpretation for the 304 AUS nodules. AUS-NA and AUS-other each accounted for 50% of the total number of nodules. In approximately half of the AUS-NA specimens, the reason for classification in this category was focal nuclear atypia, whereas it was nuclear and architectural atypia (28.9%) or extensive but mild nuclear atypia (23.0%) in the other half. Atypical cyst-lining and histiocytoid cells were not noted in any of the cases. Among 152 AUS-other nodules, 93 (61.2%) showed architectural atypia and the remaining showed oncocytic atypia (19.7%), atypical lymphoid cells (17.8%) and atypia not otherwise specified (1.3%) (one each of possible medullary thyroid carcinoma and possible parathyroid lesion). Among 27 nodules with atypical lymphoid cells, 13 nodules (48.1%) were analysed using flow cytometry, and 7 (53.8%) of these 13 nodules showed light-chain restriction. One nodule with possible parathyroid lesion was subjected to immunocytochemistry using anti-GATA-3 antibody, and a positive result confirmed the lesion.
| Cytological interpretation | Nodules |
|---|---|
| AUS with nuclear atypia | 50.0% (152) |
| Focal nuclear atypia | 48.0% (73) |
| Nuclear and architectural atypia | 28.9% (44) |
| Extensive but mild nuclear atypia | 23.0% (35) |
| Atypical cyst lining cells | 0% (0) |
| Histiocytoid cells | 0% (0) |
| AUS-other | 50.0% (152) |
| Architectural atypia | 61.2% (93) |
| Oncocytic atypia | 19.7% (30) |
| Atypical lymphoid cells | 17.8% (27) |
| Atypia not otherwise specified | 1.3% (2) |
| Possible medullary thyroid carcinoma | 1 |
| Possible parathyroid lesion | 1 |
- Abbreviation: AUS, atypia of undetermined significance.
Repeat aspiration was performed for 60 (19.7%) AUS nodules (Table 2). The repeat aspiration rates for AUS-NA and AUS-other were 25.0% and 14.5%, respectively. A significant difference was found between their frequencies (p < 0.05). Among the AUS-NA nodules, the repeat aspiration rates for AUS with focal nuclear atypia and extensive but mild nuclear atypia were 28.8% and 28.6%, respectively, whereas the rate for AUS with nuclear and architectural atypia was the lowest (15.9%). Among the AUS-other nodules, the repeat aspiration rates for AUS with architectural atypia, oncocytic atypia, atypical lymphoid cells and possible medullary thyroid carcinoma were 11.8%, 16.7%, 18.5% and 100%, respectively. Five AUS nodules with atypical lymphoid cells were repeatedly aspirated for flow cytometry, and three (60.0%) of these showed light-chain restriction. In one nodule with possible medullary thyroid carcinoma, the possibility was ruled out based on calcitonin measurement using repeatedly aspirated material.
| Cytological interpretation | Repeat aspiration rates | Periods between first and second aspirations (day) | Categories of repeat aspirations | Confirmation rate | |||||
|---|---|---|---|---|---|---|---|---|---|
| ND | BN | AUS | FN | SFM | MT | ||||
| AUS with nuclear atypia (152) | 25.0% (38) |
3–1336 (median: 16) |
3 | 15 | 10 | 1 | 2 | 7 | 57.9% (22) |
| Focal nuclear atypia (73) | 28.7% (21) |
7–1299 (median: 22) |
1 | 10 | 3 | 0 | 2 | 5 | 71.4% (15) |
| Nuclear and architectural atypia (44) | 15.9% (7) |
5–1336 (median: 8) |
1 | 1 | 3 | 1 | 0 | 1 | 28.6% (2) |
| Extensive but mild nuclear atypia (35) | 28.6% (10) |
3–595 (median: 7) |
1 | 4 | 4 | 0 | 0 | 1 | 50.0% (5) |
| AUS-other (152) | 14.5% (22) |
3–1162 (median: 19.5) |
0 | 12 | 7 | 3 | 0 | 0 | 54.5% (12) |
| Architectural atypia (93) | 11.8% (11) |
5–608 (median: 19) |
0 | 7 | 3 | 1 | 0 | 0 | 63.6% (7) |
| Oncocytic atypia (30) | 16.7% (5) |
3–37 (median: 6) |
0 | 3 | 0 | 2 | 0 | 0 | 60.0% (3) |
| Atypical lymphoid cells (27) | 18.5% (5) |
35–1162 (median: 238) |
0 | 1 | 4 | 0 | 0 | 0 | 80.0% (4) |
| Light chain restriction 3 | |||||||||
| Possible medullary thyroid carcinoma (1) | 100% (1) | 14 | 0 | 1 | 0 | 0 | 0 | 0 | 100% (1) |
| Calcitonin negative 1 | |||||||||
| Total (304) | 19.7% (60) |
3–1336 (median: 15) |
3 | 27 | 17 | 4 | 2 | 7 | 61.7% (37) |
- Abbreviations: AUS, atypia of undetermined significance; BN, benign; FN, follicular neoplasm; MT, malignant; ND, nondiagnostic; SFM, suspicious for malignancy.
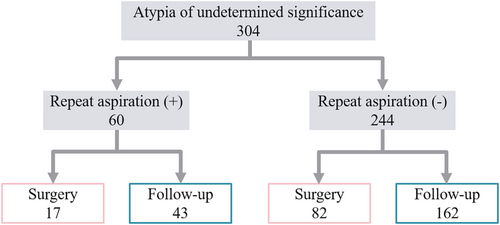
The period between the first and second aspiration ranged from 3 to 1336 days (median: 16 days). The median was the longest (238 days) for AUS with atypical lymphoid cells. The percentage of the cases found to be benign or malignant by repeat aspiration (confirmation rate) was not significantly different (p = 0.5828), being 61.7% for AUS overall, 57.9% for AUS-NA and 54.5% for AUS-other. On the contrary, AUS with atypical lymphoid cells and possible medullary thyroid carcinoma predicted based on ancillary tests showed high confirmation rates, greater than 80%. The rate of AUS with nuclear and architectural atypia was the lowest (28.6%). Seventeen nodules (28.3%) remained categorised as AUS. Four (6.7%) nodules with repeat aspiration underwent further aspiration and were categorised as benign (three cases) or FN (one case). Figure 1 shows the clinical course of the nodules with or without repeat aspiration. Among nodules with repeat aspiration, 17 (28.3%) and 43 (71.7%) were resected and followed up, respectively. Meanwhile, among the 244 nodules without repeat aspiration, 82 (33.6%) and 162 (66.4%) were resected and followed up, respectively.
Surgery was performed for 99 (32.6%) AUS nodules. Table 3 shows the histological diagnoses of resected nodules. These included 1 (1.0%) non-neoplastic lesion, 32 (32.3%) benign tumours, 26 (26.3%) low risk tumours and 39 (39.4%) malignant tumours. The remaining one nodule could not be diagnosed grossly. Low-risk tumours included 18 (69.2%) NIFTP. A total of 71.8% of malignant tumours were PTC, and the remaining were mucosa-associated lymphoid tissue (MALT) lymphoma, well-differentiated carcinoma, not otherwise specified, and follicular carcinoma.
| Histological diagnosis | Nodules |
|---|---|
| Non-neoplastic lesion | 1.0% (1) |
| Hashimoto's thyroiditis | 100% (1) |
| Benign tumour | 32.3% (32) |
| Follicular nodular disease | 50.0% (16) |
| Follicular adenoma | 46.9% (15) |
| Oncocytic adenoma | 3.1% (1) |
| Low-risk tumour | 26.3% (26) |
| Noninvasive follicular thyroid neoplasm with papillary-like nuclear features | 69.2% (18) |
| Follicular tumour of uncertain malignant potential | 19.2% (5) |
| Well-differentiated tumour of uncertain malignant potential | 11.5% (3) |
| Malignant tumour | 39.4% (39) |
| Papillary thyroid carcinoma (including 1 follicular variant) | 71.8% (28) |
| Mucosa-associated lymphoid tissue lymphoma | 15.4% (6) |
| Well-differentiated carcinoma, not otherwise specifieda | 10.3% (4) |
| Follicular carcinoma | 5.1% (2) |
| Could not be found grossly | 1.0% (1) |
- a These could not be differentiated from follicular carcinoma and papillary thyroid carcinoma owing to mild nuclear atypia.
Table 4 shows the resection rates and ROMs for AUS nodules. The resection rates for AUS-NA and AUS-other were not significantly different (p = 0.1141), being 36.8% and 28.3%, respectively. AUS with nuclear and architectural atypia had the highest (59.1%) resection rate, and 38.5% of these were NIFTP. PTC accounted for 26 (46.4%) of the 56 AUS-NA nodules. All nodules with atypical lymphoid cells were MALT lymphoma. The overall ROM for the whole AUS was 12.8%, and the ROMs for AUS-NA were significantly higher than those for AUS-other (all p < 0.0005). AUS with architectural atypia showed the lowest overall ROM (1.1%), followed by AUS with oncocytic atypia (3.3%).
| Cytological interpretation (304) | Resection rates | Resected ROM | Overall ROM |
|---|---|---|---|
| AUS with nuclear atypia (152) | 36.8% (56) | 55.4% (31) | 20.4% |
| Focal nuclear atypia (73) | 26.0% (19) | 73.7% (14) | 19.2% |
| Nuclear and architectural atypia (44) | 59.1% (26) | 42.3% (11) | 25.0% |
| Extensive but mild nuclear atypia (35) | 31.4% (11) | 54.5% (6) | 17.1% |
| AUS-other (152) | 28.3% (43) | 18.6% (8) | 5.3% |
| Architectural atypia (93) | 35.5% (33) | 3.0% (1) | 1.1% |
| Oncocytic atypia (30) | 13.3% (4) | 25.0% (1) | 3.3% |
| Atypical lymphoid cells (27) | 22.2% (6) | 100% (6) | 22.2% |
| Total (304) | 32.6% (99) | 39.4% (39) | 12.8% |
- Abbreviations: AUS, atypia of undetermined significance; ROM, risk of malignancy.
Table 5 shows the resection rates and ROMs for AUS-NA based on ultrasonography findings and repeat aspiration. Nodules with a diameter ≤ 10 mm showed significantly lower resection rate and overall ROM than those with diameter > 10 mm (p < 0.001, p < 0.05); however, the differences in resected ROMs were not statistically significant. Twelve nodules with diameter ≤ 10 mm were resected because of association with another resected nodule (five nodules), metastatic lesion (two nodules), nodular enlargement (two nodules), risk of invasion to the recurrent nerve (one nodule) or patient's wish (one nodule). Resection rate and overall ROM for the nodules with ultrasonographically low suspicion were lower than for those with intermediate to high suspicion (p < 0.05, p < 0.05). No statistically significant differences in the resection rates, resected ROM or overall ROM were found between the nodules with and without repeat aspiration.
| Resection rate | p | Resected ROM | p | Overall ROM | p | |
|---|---|---|---|---|---|---|
| Tumour size | ||||||
| ≤ 10 mm (64) | 18.8% (12) | < 0.001 | 66.7% (8) | 0.5163 | 12.5% | < 0.05 |
| > 10 mm (87) | 50.6% (44) | 52.3% (23) | 26.4% | |||
| Ultrasonography results | ||||||
| Low suspicion (50) | 20.0% (10) | < 0.005 | 30.0% (3) | 0.1583 | 6.0% | < 0.05 |
| Intermediate to high suspicion (97) | 46.4% (45) | 60.0% (27) | 27.8% | |||
| Repeat aspiration | ||||||
| Done (38) | 26.3% (10) | 0.1735 | 50.0% (5) | 0.7384 | 13.2% | 0.2496 |
| Not done (114) | 40.4% (46) | 56.5% (26) | 22.8% | |||
- Abbreviation: ROM, risk of malignancy.
4 Discussion
AUS specimens contain cells with architectural and/or nuclear atypia that is not enough to be classified as suspicious for FN, suspicious for malignancy or malignant [3]. According to TBSRTC, AUS accounts for 1%–20% of thyroid FNAC specimens, with 10% as a realistic upper limit [3]. The Japanese reporting system recommends that the frequency of AUS nodules should be less than 10% of adequate number of samples [16]. At our high-volume thyroid specialty hospital, the frequency of AUS was extremely low (4.3%), probably due to the high percentage of thyroid carcinoma patients and high quality of FNAC [12]. The overall ROM was 12.8%, slightly lower than that in TBSRTC (16%). Considering the difference in ROMs, TBSRTC recommends AUS diagnosis to subclassify the nodules into AUS-NA or AUS-other [3]. Previous reports revealed that the ROM for AUS-NA (16%–29%) was higher than that for AUS-other (6%–19%) [5, 17, 18]. Our results agreed with previous reports, supporting the relevance of the AUS subclassification. However, in TBSRTC, the clinical management is not yet based on subclassification.
In Japan, active surveillance is an option for the clinical management of low-risk PTC with size ≤ 10 mm [19], despite the indication of FNAC for thyroid nodules > 5 mm in size [11]. Hence, in our study, the AUS-NA showed a lower resection rate (36.8%) than the rates reported from other countries (50%–60%) [20, 21], and the rates were even lower (18.8%) for nodules ≤ 10 mm in size.
For AUS-NA, ultrasonography findings are important for risk stratification [22, 23]. In our study, the AUS-NA nodules with ultrasonographically low suspicion showed lower overall ROM (6.0%) than those with intermediate to high suspicion (27.8%). Therefore, clinical management of AUS-NA should vary according to ultrasonographic findings.
AUS-other presents miscellaneous scenarios, and it would be impossible to discuss the clinical management for all cases. AUS with architectural atypia and oncocytic atypia showed low overall ROMs (1.1% and 3.3%), comparable to those for the negative results in molecular testing (2%–5%) [3, 8, 24, 25]. This indicated that AUS with architectural or oncocytic atypia may be safe to monitor ultrasonographically rather than by repeat aspiration or molecular testing. Because low ROMs have also been reported [17, 18, 20, 22, 26], we inferred that these results are not specific to the present study. However, resection should be considered for nodules exhibiting features suggesting potential for malignancy, such as ultrasonographically high suspicion, tumour size > 30 mm and tumour volume-doubling rate > 1.0/year [9].
AUS nodules with atypical lymphoid cells, possible medullary thyroid carcinoma or parathyroid lesion occupy a minor population, and their frequencies have not been identified; in the present study, we determined their frequencies among AUS-other nodules to be 17.8%, 0.7% and 0.7%, respectively. Ancillary tests are useful for such cases [3, 13-15, 27-29]. For AUS with atypical lymphoid cells, flow cytometry using aspirated materials is recommended [14, 27]. The sensitivity, specificity, positive predictive value and negative predictive value are 75.0%, 88.4%, 82.8% and 82.6%, respectively [27]. In the present study, 10 of 18 nodules showed light-chain restriction in flow cytometry analysis. Some thyroid MALT lymphomas may regress spontaneously, allowing for clinical follow-up [30]. For AUS with possible medullary thyroid carcinoma, immunocytochemistry using calcitonin or measurement of calcitonin in serum or aspirated materials are recommended [13, 28, 31]. For AUS with parathyroid lesion, immunocytochemistry using GATA-3 or measurement of PTH in aspirated materials is recommended [15, 29, 31]. Immunocytochemistry can be performed without repeat aspiration by dividing the observed specimens using the cell-transferred method or by preparing additional liquid-based cytology specimens [32, 33]. In all three scenarios, ancillary tests could provide reliable preoperative diagnoses.
In the USA, where surgery is expensive, molecular testing is cost-effective in determining the clinical management of AUS. In contrast, repeat aspiration may be the first choice in countries where molecular testing is expensive or not commercially available [6, 34, 35]. In the present study, not all AUS nodules underwent repeat aspiration, with a repeat aspiration rate of 19.7%. This rate was within the range reported previously (10%–41%) [21, 24, 36, 37]. Repeat aspirations were performed more frequently for AUS-NA (25.0%) than for AUS with architectural atypia (11.8%). The confirmation rates for the repeat aspiration results were greater than 50% for all types except for AUS with nuclear and architectural atypia (28.6%). However, we did not find any significant difference in ROMs for AUS-NA between the nodules with and without repeat aspiration. Jan et al. also reported no correlation between ROM for nodules with and without repeat aspiration [38]. Therefore, repeat aspiration for AUS-NA may be recommended with limited targeting. AUS with atypical lymphoid cells and possible medullary thyroid carcinoma underwent repeat aspiration for ancillary tests and showed a high confirmation rate. We, therefore, strongly recommend repeat aspiration for these nodules.
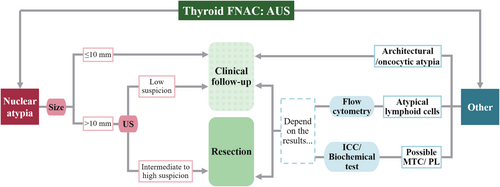
Based on the above-mentioned considerations, we created an algorithm for each subdivision based on cytological findings, as depicted in Figure 2. For AUS-NA nodules with size ≤ 10 mm, clinical follow-up is recommended. For AUS-NA nodules with size > 10 mm, clinical follow-up is recommended in cases of ultrasonographically low suspicion, and resection is recommended in cases of ultrasonographically intermediate to high suspicion. For AUS nodules with architectural or oncocytic atypia, clinical follow-up is recommended. For AUS nodules with atypical lymphoma cells, clinical management should be considered depending on the results of flow cytometry using aspirated materials. For possible medullary thyroid carcinoma or parathyroid lesion, immunocytochemistry or biochemical tests using needle washout fluid can be used for reliable preoperative diagnoses. On applying this algorithm to AUS nodules used in this study, the resected and overall ROMs for clinical follow-up and resection groups were 38.1% (16/42) and 59.3% (16/27), and 5.9% (13/222) and 22.0% (13/59), respectively. Significant differences in overall and resected ROM between the two group were found (p < 0.0001, p < 0.001). Of the 14 resected nodules that were not malignant in the group recommended for resection, 9 (64.3%) were diagnosed as NIFTP.
5 Conclusion
We propose a clinical management algorithm for each subdivision of AUS nodules based on cytological findings, repeat aspiration rates, risks of malignancy, ultrasound findings and results of ancillary tests except for molecular testing. We expect this to be useful for the clinical management of AUS nodules. While interobserver variation in the criteria for classification as AUS may make it difficult to completely adopt this algorithm at other institutions [39], we believe that some elements can be used as a reference for risk stratification in situations where molecular testing is not available.
Author Contributions
A. Suzuki and M. Hirokawa were responsible for the conception or design of the work, drafting the article, and giving final approval of the version to be published. M. Kawakami and T. Kudo contributed to data collection, data analysis, and interpretation. A. Miyauchi and T. Akamizu provided critical revision of the article.
Ethics Statement
The study protocol was reviewed and approved by the Institutional Review Board of Kuma Hospital (20231012-4) and was in accordance with the 1964 Helsinki declaration and its amendments or comparable ethical standards.
Consent
All subjects provided informed consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.