Practical issues related to immunocytochemistry on cytological smears: Tips and recommendations
Abstract
Objective
Immunocytochemistry (ICC) is essential for enhancing diagnostic accuracy and identifying markers for diagnosis, prognosis and targeted therapies. While cell blocks (CBs) are preferred for standardization and optimized staining, cytological smears are an alternative when CBs are unavailable. However, the literature on ICC protocols for smears is sparse. This review addresses preparation, fixation and protocols for nuclear and cytoplasmic antibodies on smears, drawing from our laboratory's experience.
Methods
We reviewed procedures for ICC on cytological smears using existing literature and practical insights from our laboratory.
Results
Commercially available antibodies were found to be reliable for ICC on smears if specimens are properly prepared and fixed. Protocols developed in our laboratory maintained antigenicity and provided clear staining results.
Conclusions
Although ICC on CBs is the gold standard for standardization, cytological smears are a viable alternative when CBs are unavailable. Success in ICC on smears depends on proper preparation and fixation. This review offers practical protocols and insights to help laboratories optimize ICC on cytological smears. Further research and standardization are necessary to enhance reproducibility and reliability of ICC on smears. The practical information provided is based on personal experience in our laboratory.
Graphical Abstract
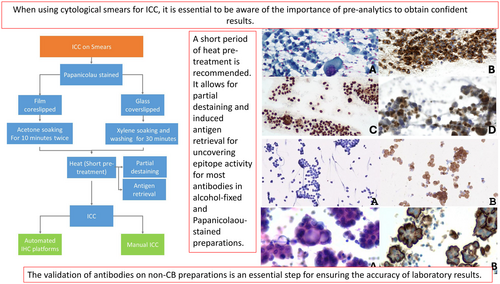
When using cytological smears for Immunocytochemistry (ICC), it is essential to be aware of the importance of pre-analytics to obtain confident results. The validation of antibodies on non-CB preparations is an essential step for ensuring the accuracy of laboratory results.
ICC is an essential ancillary technique that can be performed on cytological smears and its success relies on accurate specimen collection, handling, appropriate triage and processing. Detailing preparation, fixation and protocols for nuclear and cytoplasmic antibodies represents the focus of this review.
1 INTRODUCTION
In modern medicine, cytology serves as a primary diagnostic approach across various neoplastic and non-neoplastic conditions. It plays a critical role, particularly in advanced-stage cancer cases where cytological samples are often the only available material for diagnosis. Utilizing these samples for ancillary studies, such as immunocytochemistry (ICC) and biomarker analysis, is essential for identifying the primary tumour and guiding personalized treatment.1-3
Cytological samples include a diverse range of specimen types that are utilized in routine clinical practice. ICC is an essential ancillary technique, which is indispensable for establishing a definitive diagnosis, confirming cellular lineages, and discerning tumour origins, gradually influencing prognostic and predictive strategies on treatment.3-5
To this day, many challenges remain implicated in the validation of ICC in different cytological samples, especially in terms of varied pre-analytical conditions and processing techniques.6, 7 This article emphasizes the intricacies of ICC protocols on cytological smears, highlighting the importance of meticulous pre-analytics, standardized protocols and rigorous quality control measures. Additionally, it explores the advantages and challenges associated with the use of different cytological preparations for ICC, offering insight into optimizing ICC procedures for reliable diagnostic outcomes.
2 OVERVIEW OF ICC ON CYTOLOGICAL SAMPLES
From a clinical standpoint, cytology is a first-line diagnostic procedure in many neoplastic and non-neoplastic settings. In advanced-stage cancer patients, cytological samples may be the only material available for diagnosis, biomarker testing and selecting patients for targeted therapies.1-3
Different types of cytological samples are used in routine practice. Immunohistochemistry (IHC) is an essential ancillary technique in pathology that plays a crucial role in diagnosing, classifying and understanding various diseases. It involves using antibodies to detect specific proteins or antigens within tissue samples, allowing pathologists to visualize and analyse cellular and molecular markers. ICC consists of applying immunohistochemistry techniques to cytological specimens. Efficient implementation of ICC is crucial in the cytological diagnosis of benign and malignant lesions, owing to cytopathology's remarkable development and applications.2-5, 8, 9 As an adjunct to cytomorphology, ICC is a useful ancillary tool for establishing a definitive diagnosis, confirming the cell lineage, and/or determining the site of origin of a malignancy. It is also increasingly used for prognostic/predictive purposes, therapeutic guides and interventions.
IHC is one of the standard methods used for predictive biomarker testing. It is cost-effective and readily available in most laboratories. IHC has a quick turnaround time and can be performed using a relatively smaller number of tumour cells. It is also less technically challenging when compared to other molecular and cytogenetic methods. This makes it a standard and preferred method for biomarker testing. While most predictive IHC assays are validated primarily on formalin-fixed paraffin-embedded (FFPE) histologic tissue samples, a significant fraction of cancer patients, especially NSCLC patients, are diagnosed using cytology samples, resulting in an increasing demand for predictive biomarker testing on cytologic specimens.10, 11 According to the IASLC Pathology Committee, all cytologic preparations, including cell blocks (CBs), ethanol-fixed and air-dried slides, can be used for ICC.12
However, cytologic specimens pose a more significant challenge for validation.13, 14 This is partly due to the wide variety of cytologic specimen preparations comprising multiple pre-analytic variables, including a variety of collection media, preservatives, fixatives, storage conditions, processing techniques and stains, among others.9, 15 Non-CB cytologic preparations, including air-dried and alcohol-fixed direct smears, cytospins and liquid-based cytology (LBC) preparations, pose a more significant challenge for ICC validation. Of these, immunostaining of ethanol-fixed smears or cytospins is used more frequently, with prior Papanicolaou staining that allow identifying areas or cells of interest. Air-dried, unfixed extra slides can also be utilized for ICC, usually after a post-fixation step involving formalin or acetone.14 ICC on cytological smears or LBC requires careful comparative validation with formalin-fixed specimens.
While several professional organizations have issued recommendations for the use of cytological specimens for ancillary testing in NSCLC samples,4, 12, 15-17 specific guidelines for assay validation for ICC on cytology samples (for instance, number of samples and selection of markers) are not available. Their choice is typically left at the discretion of the experienced cytopathologist and laboratories.18, 19 Moreover, there is a general lack of information about practical technical viewpoints and protocols for performing ICC on smears, not only for biomarkers but also for assuring a definitive diagnosis, confirming the cell lineage and/or determining the site of origin of a tumour.
All the information shown in this paper comes from our personal experience and the protocols were developed in our laboratory by a team of one cytopathologist (MDL) and four technicians (JG, VO, NG and NF).
3 THE RATIONALE FOR THE USE OF SMEARS
Although CBs are the preferred and recommended cytological sample to perform ICC for the reasons mentioned above, in some circumstances, they are not available or lack cellularity. If so, other types of cytological samples, including smears, can come to the rescue. It is essential to be aware of the importance of pre-analytics to get confident results.
4 HOW TO HANDLE SMEARS FOR ICC: PRE-ANALYTICS, PROTOCOLS AND CONTROLS
In addition to CBs, other cytopathology preparations that can be used for ICC include alcohol-fixed direct smears, cytospins and LBC. There are references in the literature that demonstrate the use of smears for ICC.20 However, no detailed procedures or technical recommendations are available. Three critical factors must be considered: Pre-analytics, Protocols and Controls.
5 PRE-ANALYTICS
The success of this procedure relies on accurate specimen collection, handling, appropriate triage of the samples and processing.11, 13 The role of the trained cytotechnicians is extremely important. Rapid on-site evaluation (ROSE) by a cytopathologist or a trained cytotechnologist is highly recommended in all fine needle aspiration (FNA) procedures. ROSE has advantages such as higher yield, the need for fewer passes and cost-effectiveness, the control of pre-analytics and proper triage for ancillary studies, reasons why including it is strongly recommended.14
The role of skilled technicians is vital. A uniform, well-spread sample with minimal obscuring material is a prerequisite for reliable ICC staining. Immediate alcohol fixation is mandatory. The smears can lose antigenicity rapidly if stored as air-dried slides without post-fixation.18 There are references in the literature that indicate that both Pap-stained and Giemsa-stained slides (coverslipped) can be used.18, 21
In our experience, we prefer Papanicolaou-stained smears because most antibodies, including nuclear, cytoplasmic and cytoplasmic membrane antigen antibodies, work well with this technique. Nuclear antigen antibodies also work in Diff-Quik stained smears, but our experience with Diff-Quik smears is limited (personal observation). The storage time is not well established. Some authors consider ICC smears stained for up to 2 years to be optimal.16
Unlike paraffin sections, Papanicolaou-stained and coverslipped smears retain antigenicity. As an example, we successfully performed ICC with AC against PD-L1 in smears from placenta used as controls aged more than 2 years old. Coverslipped smears do not lose antigenicity, and archived smears can be used reliably. Soaking in xylene (cristal coverslip) or Acetone (plastic coverslip) to remove the mounting medium has no negative effect either.
While studies in the literature suggest that some fixatives can alter the antigenicity and ICC results in cytologic samples, a report from the United Kingdom National External Quality Assessment Service (UK NEQAS) suggests that all non-formalin fixatives, including methanol, and excluding acetone, yield a quality of immunostaining comparable to that of formalin fixation alone.22
Additionally, if we happen to run out of smears and need to test for another antibody, we often use a smear that was previously stained with an antibody and had negative results. This can be done by following the same protocol employed with Papanicolaou-stained smears.
6 PROTOCOLS
The validation of antibodies on non-CB preparations is an essential step for ensuring the accuracy of laboratory results. In this regard, laboratory validation must be conducted using imprints derived from biopsies or surgical specimens. The comparison of results, until confidence is established with each antibody, is equally important. To warrant accurate and reliable laboratory results, it is critical that laboratory personnel follow these guidelines for a thorough and comprehensive validation process. To further guarantee the quality of laboratory results, external quality controls are very much desirable.
To remove the coverslip, we follow a two-step process. If the smears are coverslipped with film, we soak them in acetone for 10 min twice. In the case of glass coverslips, we soak the slides in Xylene until the glass comes off easily without applying any force. Finally, we wash the slides with clean xylene for 30 min to ensure the complete removal of any residue. The majority of antigens do not require retrieval in alcohol-fixed smears. This is mainly because alcohol-based fixatives work by causing tissue dehydration and protein coagulation.14, 18 Protease antigen retrieval should be avoided. However, a short period of heat pre-treatment is recommended. It allows for partial destaining and induced antigen retrieval for uncovering epitope activity for most antibodies in alcohol-fixed and Papanicolaou-stained preparations. (Table 1).
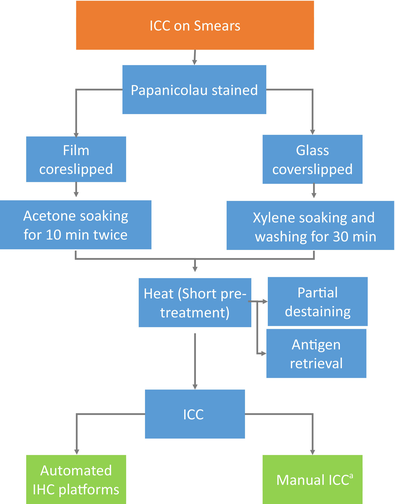
- a In our laboratory, most of the ICC performed on cytological smears is done on automated IHC platforms.
Immunostaining can be performed either manually or using automated immunostainers. Manual staining is less standardized and operator-dependent. Our laboratory is equipped with two immunostaining platforms: BENCHMARK ULTRA-VENTANA-ROCHE—and AUTOSTAINER LINK 48-Agilent, Dako. All the antibodies we use on smears were validated for both platforms. However, we use one or the other depending on the antibody. Tables 2 and 3 show the protocols used with the two commercial automated platforms for some of the most used antibodies in our daily practice.
| Antibody | Clon | Vendor | Platform | Antigen retrieval | Detection kit | Antibody incubation time |
|---|---|---|---|---|---|---|
| CK Cam5.2 | Cam 5.2 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) | 95°C CC1 (PH9) 36′ | ULTRAVIEW | 44′ |
| CK Cam5.2 | Cam 5.2 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ + AMPLIFICATOR |
| CK7 | SP52 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
ULTRAVIEW | 16′ |
| CK7 | OV-TL 12/30 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| TTF1a | SP141 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
ULTRAVIEW | 36′ |
| P40 | BC28 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
OPTIVIEW | 48′ |
| P40 | P | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 8′ | ENVISION | 40′ + AMPLIFICATOR |
| PD-L1 SP263 | SP263 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
CC1 (PH9) 100°C 40′ |
OPTIVIEW | 16′ 36°C |
| PD-L1 22c3 | 22c3 | AGILENT | AUTOSTAINER LINK 48 | 97°C PH 6 20′ | ENVISION PD-L1 22c3 | 30′ + AMPLIFICATOR |
| GATA3 | L50-823 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
ULTRAVIEW | 32′ |
| GATA3 | HG3-31 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| Oestrogen receptor | ER(SP1) | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 36′ |
ULTRAVIEW | 32′ |
| Oestrogen receptor | Ep1 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| E-cadherin | 36 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 36′ |
ULTRAVIEW | 32′ |
| E-cadherin | 4A2C7 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ + AMPLIFICATOR |
| Ki-67 | Ki (30–9) | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 36′ |
ULTRAVIEW | 32′ |
| Ki-67 | MIB-1 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| PAX-8a | MRQ-50 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
ULTRAVIEW | 32′ |
| NKX3.1a | EP356 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
ULTRAVIEW | 32′ |
| TRPS1a | POLICLONAL | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 52′ |
ULTRAVIEW | 48′ |
| CALRETININ | SP65 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 36′ |
ULTRAVIEW | 16′ |
| CALRETININ | DAK-Calret 1 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| D2-40 | D240 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 36′ |
ULTRAVIEW | 36′ |
| D2-40 | D240 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| WT1 | 6F-H2 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
ULTRAVIEW |
32′ |
| WT1 | 6F-H2 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ + AMPLIFICADOR |
- a TTF1, PAX-8, NKX3.1 and TRPS1 are not performed on AGILENT.
| Antibody | Clon | Vendor | Platform | Antigen retrieval | Detection kit | Antibody incubation time |
|---|---|---|---|---|---|---|
| CD3 | 2GV6 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 52′ |
ULTRAVIEW | 32′ |
| CD3 | POLICLONAL | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ + AMPLIFICATOR |
| CD20 | L26 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 36′ |
ULTRAVIEW | 32′ |
| CD20 | L26 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| CD30a | Ber-H2 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ + AMPLIFICATOR |
| CD45 (LCA) | RP2/18 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 36′ |
ULTRAVIEW | 32′ |
| CD45 (LCA) | LCA-RP | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| C-KITa | POLICLONAL | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| DOG-1b | SP31 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
OPTIVIEW | 52′ |
| CD123b | 6H6 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 52′ |
ULTRAVIEW | 44′ |
| HMB45 | HMB45 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 8′ |
ULTRAVIEW | 24′ |
| HMB45 | HMB45 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| MELAN A | A103 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
ULTRAVIEW | 36′ |
| MELAN A | A103 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| SOX10 | SP267 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 52′ |
ULTRAVIEW | 32′ |
| SOX10 | POLICLONAL | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ + AMPLIFICATOR |
| S100 | 4C4.9 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
92°C CC1 (PH9) 20′ |
ULTRAVIEW | 44′ |
| S100 | POLICLONAL | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
| MYOD1b | EP212 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 64′ |
ULTRAVIEW | 32′ |
| TLE-1b | 1F5 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 52′ |
ULTRAVIEW | 44′ |
| VIMENTIN | V9 | ROCHE | BENCHMARK ULTRA (VENTANA-ROCHE) |
95°C CC1 (PH9) 36′ |
ULTRAVIEW | 16′ |
| VIMENTIN | V9 | AGILENT | AUTOSTAINER LINK 48 | 98°C PH 9 5′ | ENVISION | 20′ |
- a CD30 and C-KIT are not performed on VENTANA.
- b DOG-1, CD123, MYOD1 and TLE-1 are not performed on AGILENT.
Automated IHC platforms are used according to manufacturers' instructions. If the quality of the ICC staining in smears is not satisfactory using the same procedures as in FFPE samples, we make minor adjustments to the automated protocol. This involves slight modifications in the pH and the incubation time of the specific antibody in question.
In our laboratory, we scan the Papanicolaou-stained smears before using them for ICC.
Figures 1-8 show several examples with different commonly used antibodies.
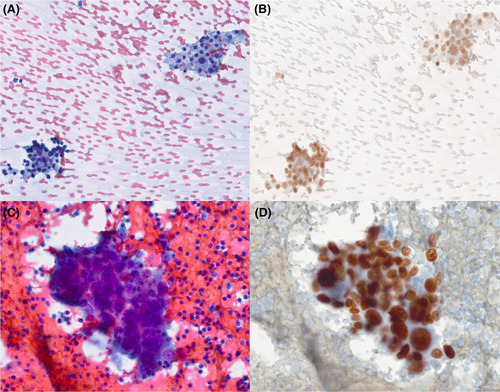
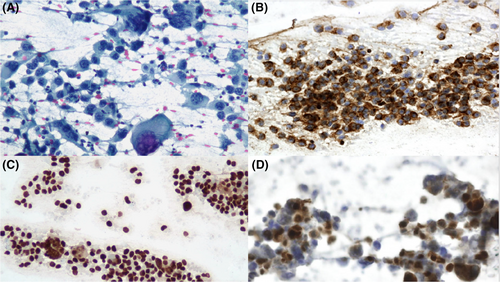
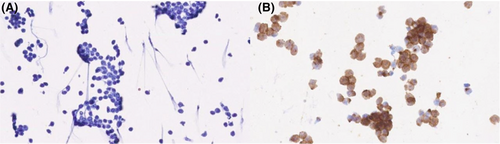
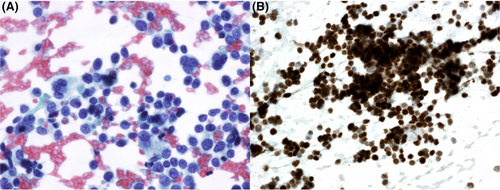
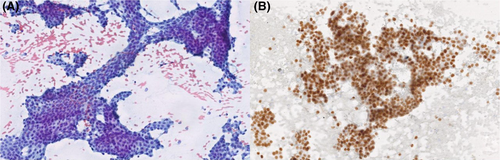
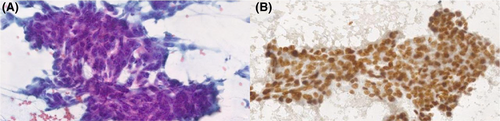
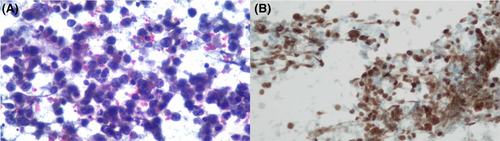
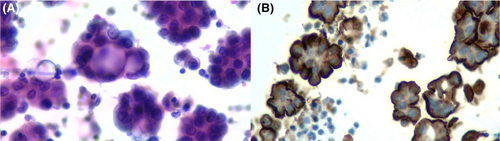
7 CONTROLS
The controls can be collected from smears, cytospins or direct imprints obtained from surgical bench macroscopic specimens or biopsies. Well-characterized in vitro cell lines can be used as well. The control slides can be stained and coverslipped and/or refrigerated or stored at −20°C to preserve the antigens. Only after the antibodies are correctly validated can FFPE controls be used. In our laboratory, we use FFPE controls except with PD-L1, where we use Papanicolaou-stained smears from the placenta.
8 CONCLUSIONS
When CBs are unavailable or lack cellularity, smears are preferred in our laboratory for ICC over other types of cytological samples. Contrary to what has been noted, alcohol fixative does not interfere with antigenicity, at least with the most frequently used antibodies. Using Papanicolaou-stained smears allows us to evaluate of the exact cells we want to analyse. Scanning the smears is highly recommended, or if not possible, pictures of the cells must be taken before dedicating a given smear to ICC.
Although negative smears can be reused for ICC, the main disadvantage is the limitation of adequate smears, which makes it necessary to select the antibody panel judiciously. A rigorous internal validation is mandatory. An external quality control is needed.
AUTHOR CONTRIBUTIONS
Maria D. Lozano help in conceptualization, planification, team coordination and writing of the main text. Development of tables, graphics and figures. Allan Argueta and Ramón Robledano helped in the development of main text, table, graphics and figures. Jaione García and Vanessa Ocon help in the development of protocol preparations and tables of ICC on smears. Nerea Gómez and Nassira Fernandez helped in the development and handling of cytological smears.
ACKNOWLEDGEMENTS
The main author would like to thank the other authors, especially the technical staff, for their dedication, enthusiasm, initiative and professionalism during the years of developing these techniques.
CONFLICT OF INTEREST STATEMENT
All of the authors on this text state have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.