Quiz case: Abnormal haematopoietic cells in pleural effusion
Graphical Abstract
This case was presented because of the number of plasmablasts in a patient with a medical history of multiple myeloma. Flow cytometry is a “gold standard” technique for the diagnosis of haematological malignancies. This technique works for all fluids and should be performed in effusions (pleural, pericardial, ascites) in cases of suspected haematological malignancy. Alternatively, immunohistochemistry using appropriate markers could be performed if flow cytometry is not available.
This case illustrates a pleural infiltration by plasmablasts. Myelomatous cells were characterised by immunocytochemistry and flow cytometry.
1 CASE HISTORY
A 66-year-old non-smoking man presented to the emergency department with dyspnea and severe chest pain. He had had bronchitis for the past 15 days, and had been treated with antibiotics and corticoids without improvement. The computed tomography scan showed a massive pleural effusion on the left side. The pleural effusion was punctured and a cytological analysis performed. In the patient's medical history, there is multiple myeloma in remission for 2 years, sarcoidosis, and type 2 diabetes.
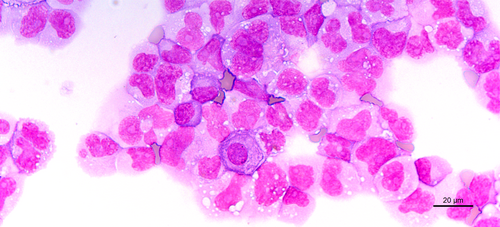
The cytological picture is shown in Figure 1.
2 MORPHOLOGY QUIZ
- Reactive mesothelial cells.
- Mesothelioma.
- Adenocarcinoma.
- Haematological malignancy.
- Immunocytochemistry using BerEP4 antibody.
- Immunocytochemistry using WT1 antibody.
- Immunocytochemistry using CD45, CD19, CD3, CD38, and CD138 antibodies.
- Flow cytometry analysis.
- High grade lymphoma.
- Hodgkin lymphoma.
- Multiple myeloma with plasmablasts.
- Acute lymphocytic leukaemia.

ANSWERS TO THE QUIZ
- The nature of the chromatin staining, the deep blue cytoplasmic staining, and the large and multiple nucleoli point to a haematological malignancy (D).
- Flow cytometry and immunocytochemistry using CD45, CD19, CD3, CD38, and CD138 can characterise these haematopoietic cells (C).
- CD45 positivity confirms the presence of haematopoietic cells. CD138 and CD38 positivity associated with kappa monoclonal expression confirm the presence of plasma cells and support a diagnosis of multiple myeloma relapse (C).
3 EXPLANATION
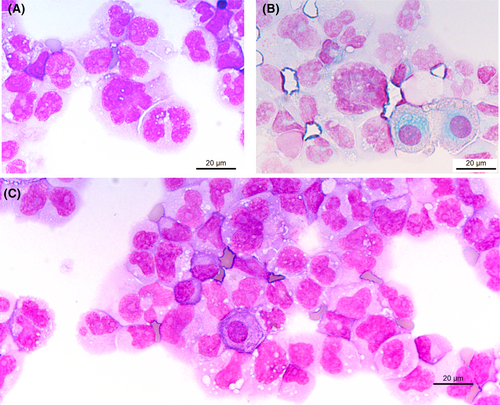
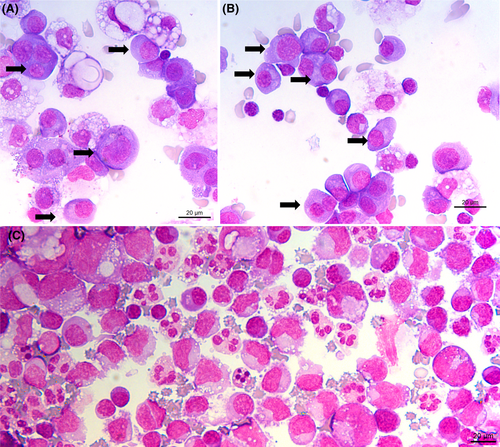
Cytological examination points to a haematological disease: one or more prominent nucleoli, chromatin appearance, and the deep blue staining of the cytoplasm. For comparison, mesothelial cells are indicated by an asterisk * in Figure 4 (corresponding to Figure 1, with annotation). Typical myelomatous plasma cells are shown in Figure 3A,B (black arrows). Plasma cells are round to ovoid with a round eccentric nucleus, clock face or spoke wheel chromatin with a nucleolus, an extensive deep blue staining cytoplasm with a perinuclear clear zone corresponding to the Golgi apparatus.1 Myelomatous plasma cells show morphological abnormalities in 50% of cases. Nuclear abnormalities include diffuse chromatin pattern, increased nucleocytoplasmic ratio, prominent nucleolus, anisokaryosis, and nuclear irregularity (including blebs, multilobulated nucleus, irregular nuclear contour, or clover-leaf shaped nucleus). Plasmablasts are immature plasma cells characterised by a large nucleus, nucleocytoplasmic ratio of >0.6, finely dispersed chromatin with a prominent nucleolus, and no perinuclear clear zone. These cytological criteria correspond to immaturity or aggressiveness of plasma cells.2
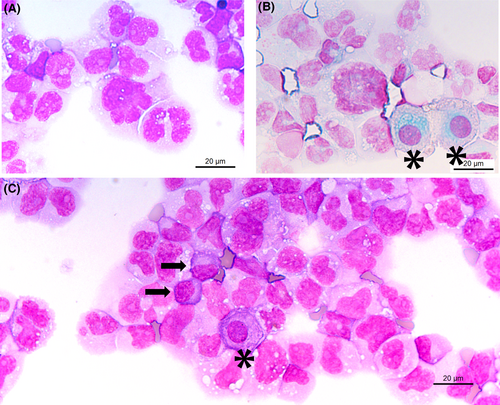
Both immunocytochemistry with appropriate markers or flow cytometry can be performed to confirm the origin of malignant cells.3 Plasma cells are usually detected by flow cytometry using the following markers: CD45, CD138, CD38, CD56, CD34, CD19, and CD117, and kappa and lambda light chains.4 Myelomatous plasma cells are characterised by negative CD19 expression (>95% of cases), weak or negative CD45 expression (70%-45% of cases), kappa or lambda cytoplasmic restriction, and moderate to strong CD56 expression (60%-80% of cases).5 In the presented case, malignant cells were CD45 weak, CD138+, CD38+, kappa light chain (99.97% of plasma cells), CD200+, CD27+, CD56− and CD19−. If flow cytometry cannot be performed, immunohistochemistry is useful using CD138, MUM1 (Multiple Myeloma Oncogene 1), kappa and lambda light chains.
In this case, the cytology was atypical, and flow cytometry was performed to confirm the nature of malignant cells. The absence of spoke wheel chromatin, the elevated nucleocytoplasmic ratio, the multiple nucleoli, and the clover-leaf shaped nuclei tend to classify these abnormal cells more as plasmablasts rather than plasma cells. The plasmablastic morphology can be seen at diagnosis but is more commonly observed when plasmablast transformation occurs in known cases of plasma cell myeloma. The presence of plasmablasts is a poor prognostic factor associated with high tumour proliferation and shortened survival.6
A differential diagnosis to plasma cell neoplasm with plasmablasts is plasmablastic lymphoma.7 It is a highly aggressive B cell non-Hodgkin lymphoma frequently associated with human immunodeficiency virus (HIV) infection or other immunodeficiencies. It represents 1% of large B cell lymphomas and 2% of all HIV-related lymphomas. Plasmablastic lymphoma cells express CD79a, MUM1, BLIMP1, XBP1, CD38, and CD138. They are negative for CD19, CD20, and PAX5.3, 8 Features favouring plasmablastic lymphoma are association with HIV, whereas features favouring multiple myeloma are monoclonal paraproteinemia, hypercalcemia, lytic bone lesions, and renal dysfunction. Another differential diagnosis is large B cell lymphoma with plasmacytoid morphology (Figure 3C). In this case B cell markers such as CD19, CD20, or PAX5 are positive. In the case presented, the medical history helped to orient the diagnosis.
Myelomatous pleural effusion (MPE) is a form of extramedullary disease with infiltration of the pleural cavity by plasma cells. It occurs in 1% to 2% of patients with multiple myeloma and is predominantly a unilateral effusion.9, 10 It can be present at diagnosis or during follow-up. As for complex karyotypic abnormalities, MPE in patients with multiple myeloma is associated with high-risk disease. MPE is associated with poor prognosis and short survival times, with a median between 2.4 to 13 months.9, 11, 12
AUTHOR CONTRIBUTIONS
Diane Frankel and Sylvie Cointe: Data collection. Diane Frankel: Writing the manuscript. Elise Kaspi, Patrice Roll, Sylvie Cointe, and Bruno Valentin: Reviewing the manuscript.
ACKNOWLEDGEMENTS
We thank Michael Mitchell for his contribution.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
INFORMED CONSENT
The patient described in this report gave his informed consent to the anonymous publication of our clinical findings in his case.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.