COVID-19 post-vaccination lymphadenopathy: A review of the use of fine needle aspiration cytology
Abstract
COVID-19 vaccine-associated clinical lymphadenopathy (C19-LAP) and subclinical lymphadenopathy (SLDI), which are mainly detected by 18F-FDG PET-CT, have been observed after the introduction of RNA-based vaccines during the pandemic. Lymph node (LN) fine needle aspiration cytology (FNAC) has been used to diagnose single cases or small series of SLDI and C19-LAP. In this review, clinical and LN-FNAC features of SLDI and C19-LAP are reported and compared to non-Covid (NC)-LAP. A search for studies on C19-LAP and SLDI histopathology and cytopathology was performed on PubMed and Google Scholar, on 11 January 2023. Reports on LN-FNAC of C19-LAP were retrieved. A total of 14 reports, plus one unpublished case of C19-LAP observed in our institution, diagnosed by LN-FNAC were included in a pooled analysis and compared to the corresponding histopathological reports. In total, 26 cases were included in this review, with a mean age of 50.5 years. Twenty-one lymphadenopathies assessed by LN-FNAC were diagnosed as benign, and three cases as atypical lymphoid hyperplasia; the latter were subsequently confirmed as benign (one by repetition of LN-FNAC, two by histological control). One case of mediastinal lymphadenopathy in a patient suffering from melanoma was reported as reactive granulomatous inflammation, while one unsuspected case was diagnosed as metastasis from melanoma. In all cases, the cytological diagnoses were confirmed by follow-up or excisional biopsy. The high diagnostic value of LN-FNAC in excluding malignant processes was extremely useful in this context and may be particularly valuable when CNB or histological excisions are difficult to perform, as was the case during Covid lockdowns.
Graphical Abstract
Lymph node (LN) Fine-needle aspiration cytology (FNAC) has been used to diagnose single cases or small series of C19-LAP. All the cytological diagnoses were confirmed by follow-up or excisional biopsy. The high diagnostic value of LN-FNAC was extremely useful during the pandemic and may be precious when CNB or histological excisions are difficult to perform as it happened during Covid lockdowns.
1 INTRODUCTION
Pandemics have occurred periodically throughout human history, but the COVID-19 pandemic had unique features in terms of its diffusion and direct and indirect effects. Unprecedented levels of investment produced, in record time, different vaccines that were used in mass vaccination programs and were administered in different countries with different modalities and different levels of efficacy among the various populations.1, 2 COVID-19 vaccines have been demonstrated to be safe and effective; however, some adverse effects have been reported.1, 2 Different COVID-19 vaccines have been developed,2, 3 and most of them are administered by intramuscular injection in alternate arms2 at determined intervals via at least two shots. As with any other vaccine,4-14 the COVID-19 vaccine may cause side effects,1, 15 the most common being local pain and inflammation at the injection site, followed by headache, fatigue, chills, myalgia, arthralgia, fever, dizziness, nausea, and vomiting, often registered after the first administration; these effects depend on the vaccine type and individual responsivity. COVID-19 vaccine-associated lymphadenopathy (C19-LAP) may also occur, mostly in axillary, clavicular or cervical lymph nodes, in the arm of vaccine inoculation. In the Pfizer BioNTech COVID-19 vaccine trial, axillary and supraclavicular C19-LAP occurring on the same side as the injection was observed in 0.3% of the vaccine group vs less than 0.1% of the placebo group.15-17 In Moderna's case, the incidence was 1.1%.15, 17 The site of the lymphadenopathy was axillary in 11% of the patients after the first dose and 16% after the second dose of the Moderna vaccine; similar findings have been reported for the Comirnaty-Pfizer/BioNTech vaccine.18, 19 It is likely that C19-LAP is merely the epiphenomenon of lymph node reactivity, since COVID-19 vaccine-associated subclinical lymphadenopathy (SLDI) has been reported in a higher percentage of cases than C19-LAP.17 The detection of C19-LAP is mainly clinical and can be confirmed by ultrasound (US). Combined clinical data and US features allow a diagnosis of reactive SLDI or C19-LAP to be made; hence, a clinical follow-up is all that is required in most cases. Nonetheless, some C19-LAP and SLDI cases with equivocal presentation or in specific clinical contexts may raise differential diagnostic problems with lymph node metastases or lymphoma.20-26 In these cases, a cytological or histological evaluation of the lymphadenopathy may be necessary. Fine needle aspiration cytology (FNAC) is routinely utilised in the diagnosis of lymphadenopathy. Numerous studies have reported that lymph node fine needle aspiration cytology (LN-FNAC) is an accurate, safe, effective, and well-tolerated diagnostic procedure, both for reactive processes and for lymphoma and metastases.5, 27-34 A review of studies on LN-FNAC in C19-LAP is reported herein.
2 MATERIALS AND METHODS
A literature search was initially performed through PubMed and Google Scholar, on 11 January 2023, using the following keywords: “COVID-19”, “vaccine”, “lymphadenopathy”, “cytology”, and “fine-needle aspiration”. No restrictions were placed on the year of publication. Only literature published in English was selected, including studies that reported histopathological and/or cytological findings in vaccine-related lymphadenopathy.17, 20, 22, 23, 35-64 Studies on SLDI, which was generally detected by 18F-FDG PET-CT, were also selected and used for the general comprehension and description of the phenomenon and to retrieve cases evaluated by histology or LN-FNAC.65-68 Recommendation articles, protocols, commentaries, and non-English articles were not considered. Data extracted from studies regarding LN-FNAC of C19-LAP and SLDI included the following: type of publication, number of patients and clinical data, type and dose of administered vaccine, delay from last vaccination to lymphadenopathy, LN site and size, cytological features and diagnosis, management and outcome. In studies reporting both LN-FNAC and histological evaluation by core needle biopsy (CNB) or surgical excision, only LN-FNAC cases were utilized for the present analysis. Finally, an unpublished case observed at our institution that met the same inclusion criteria was also added.
2.1 COVID-19 vaccines and lymphadenopathy
Almost all COVID-19 vaccines may cause reactive lymphadenopathy; most cases are subclinical and detectable only instrumentally, whereas a minority of cases show overt clinical C19-LAP. Increased lymph node 18F-FDG uptake has been detected in up to 36% of vaccinated patients69 up to 10 weeks after vaccination, with women and people aged over 65 years being most frequently affected.69, 70 Lymph node enlargement occurs less frequently; different studies have reported an incidence of ~1% among those vaccinated against COVID-1915, 16, 35, 58 depending on the specific vaccine; namely, it has been reported in 0.3% of Pfizer-BioNTech and 1.1% of Moderna vaccines, respectively58 (Table 1). While most cases of SLDI do not show clinically evident enlargement of lymph nodes, an awareness of SLDI among doctors is of fundamental importance in a variety of clinical contexts, particularly with regard to cancer staging and follow-up to avoid the risk of overdiagnosis. In our research, surgical excision or CNB of C19-LAP- or SLDI-enlarged lymph nodes have been performed in 55 cases.20, 22, 23, 35, 39, 41-43, 47, 49-63 The histological diagnoses were as follows: negative for neoplasia, reactive hyperplasia, and florid reactive hyperplasia (39 cases),23, 39, 50-61 progressive transformation of germinal centres (1 case),54 atypical follicular hyperplasia with light chain-restricted germinal centres (1 case)62 granulomatous reaction (2 cases),50, 63 metastases (4 cases),20, 22, 50 Kikuchi-Fujimoto disease (KFD; 6 cases),40-42, 49, 56, 64 Rosai-Dorfman-Destombes disease (RDD; 1 case),48 and Langerhans cell histiocytosis (LCH; 1 case).57 In cases of reactive hyperplasia, the histological features were described as a preserved lymph node structure with cortical follicular hyperplasia, enlarged germinal centres and interfollicular expansion by small lymphocytes. Prominent germinal centres and tingible-body macrophages were frequently reported. In expanded interfollicular regions, capillaries with focally prominent endothelial cells have been described.55 Diffuse or partial capsule thickness was frequently reported. The immunohistochemical phenotype (IHC) was reported in eight cases.54, 58, 61, 62, 71 The results of flow cytometry were also reported in one case.44 A case of atypical follicular hyperplasia with light chain-restricted germinal centres after a COVID-19 booster was described,62 but the specific vaccine was not reported. A case of C19-LAP with eosinophilic abscesses has also been reported.43 The histological features showed eosinophil-rich inflammation with micro-abscesses.43 COVID-19 cases associated with KFD and RDD have been described as exhibiting the typical histological features of the corresponding, non-vaccine-related entities.40-42, 48, 56, 64
| Name | Manufacturer | Type | Preparation | Dosage | Lymphadenopathy/incidence |
|---|---|---|---|---|---|
| Comirnaty | Pfizer Inc & BioNTech | mRNA | S-protein | 2 doses, 21 days apart | Yes/3%-9%58 |
| mRNA-1273 | Moderna | mRNA | S-protein | 2 doses, 28 days apart | Yes/1%-16% https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html. |
| COVID-19 Vaccine Janssen | Johnson & Johnson | Viral Vector Vaccine | Type 26 human adenovirus | COVID-19 Vaccine Janssen | Yes/Not reported70, 76 |
| COVID-19 vaccine (AZD1222) | Oxford/AstraZeneca | Viral vector vaccine | Adenovirus vector | 2 doses, 28-84 days apart | Yes <1%58 |
| Vaxzevria | Oxford/AstraZeneca | Modified adenovirus | Modified adenovirus | 2 doses, 28-84 days apart | Yes/4 cases58, 61 |
| CoronaVac | Sinovac | Inactivated virus | Whole virus inactivated | 2 doses, 28 days apart | Not available |
| Covaxin | Bharat Biotech | Viral vector vaccine | S-protein | 2 doses, 28 days apart | Not available |
| Covishield | Oxford/AstraZeneca | Viral vector vaccine | S-protein | 2 doses, 3 months apart | Not available |
| Nuvaxovid | Novavax | Protein subunit | Long S-protein | 2 doses, 21 days apart | Not available |
2.2 Fine needle aspiration cytology in cases of SLDI and C19-LAP
The present study is a pooled review based on 14 reports20-22, 35-38, 44-47, 52, 72 and one unpublished case observed by the authors. In total, 26 cases are reported, including 17 (65%) female patients, 8 (32%) male patients, and one patient whose gender was not reported,21 with an age range of 27-70 years. Nine cases (34%) had a previous or active history of malignancy, including breast cancer, papillary thyroid carcinoma, renal cell carcinoma, melanoma or lung carcinoma; one case had a positive family history of breast carcinoma. C19-LAP was reported after the first or second administrations of two types of vaccines, namely Pfizer-BioNTech (18 cases, 72%) and Moderna (5 cases, 20%); the vaccine type was reported as mRNA COVID-19 vaccine in 2 cases (8%). C19-LAP was observed in imaging examinations after the first and second administrations of the Pfizer-BioNTech vaccine, with a median delay of 10.5 days (range, 5-18 days) and 5 days (range, 1-7 days), respectively.
Lymphadenopathies were developed on the same side as the vaccine shot in 13 cases, and on the opposite side in 3 cases; the side on which the lymphadenopathy occurred was not reported in 10 cases. The specific lymph nodes reported were supraclavicular (10 cases), cervical (6 cases), axillary (7 cases), mediastinal (1 case) and submandibular (2 cases) (Table 2). All lymph nodes were evaluated using US, and were reported as enlarged and oval, with major diameters ranging from 5 to 50 mm (mean 23.7 mm) (Figure 1); spherical shape was reported in one case (Hagen et al.38). Another reported US feature was diffuse or focal cortical thickening and preserved, visible hilum in almost all cases; effaced hilum was reported in one case (Hagen et al.38). The 18F-FDG-PET/CT data were not reported for any of these cases.
| Reference | Case # | Dose, Vaccine, Onset (days) | Age/sex | Clinical history | Lymph node site/size | Ultrasound | Cytological features | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Aalbers1 | 1 | 1st, Moderna, 63 | 74/NR | Stage IV renal carcinoma | Axillary, left/23 mm | Oval, hypo-echoic, hilum present, cortical thickening | Reactive, no metastasis | Clinical |
| Trikannad et al.59 | 1 | 1st, Pfizer Inc & BioNTech, 21 | 57/F | Melanoma | Mediastinal/23 mm | NR | Granulomas, reactive changes, no evidence of malignancy | Clinical |
| Gullotti et al.20 | 1 | 2nd, Pfizer Inc & BioNTech, NR | 53/M | Negative | Axillary left/50 mm | Roundish, hypoechoic, | Metastasis melanoma | Clinical |
| Heaven et al.44 | 1 | 1st, Pfizer Inc & BioNTech, 4 | 42/M | Negative | Supraclavicular/NR | NR | Reactive hyperplasia, flow cytometry: polyclonal | Clinical, negative |
| 1 | 2nd, Pfizer Inc & BioNTech, 76 | 70/F | Psoriatic arthritis | Supraclavicular left/10 mm | NR | Reactive, large, atypical cells of uncertain significance | Repeated FNAC, negative | |
| 1 | 1st, Pfizer Inc & BioNTech, 31 | 45/M | Negative | Left submandibular/NR | NR | Atypical lymphocytes, lymphoproliferative disorder | Biopsy, negative, flow cytometry: polyclonal | |
| Garcia-Molina36 | 1 | 1st, Pfizer Inc & BioNTech, 5 | 34/F | NR | Supraclavicular/NR | Ovoid, cortical homogeneous echogenicity, Doppler increased vascularization | Polymorphous smears, some nuclei bilobed, with large nucleoli hyperchromatic. Histiocytes, no eosinophils, no mitosis. Nonspecific chronic lymphadenitis | Complete resolution after anti-inflammatory therapy |
| 1 | 1st, Pfizer Inc & BioNTech, 5 | 27/F | NR | Axillary/NR | NR | |||
| Dirven et al.72 | 1 | 1st, Pfizer Inc & BioNTech, 21 | 60/F | MEN1 | Axillary/NR | NR | Benign reactive pattern without metastatic disease | Clinical, complete resolution |
| Tan et al.37 | 1 | 1st, Pfizer Inc & BioNTech, 1 | 34/M | Negative | Supraclavicular left/11 mm | Oval, hilum not clearly visualised | Follicular hyperplasia, tingible-body macrophages | Clinical, complete resolution |
| Cardoso et al.45 | 1 | 1st, Pfizer Inc & BioNTech, 14 | 48/F | Family history of breast cancer | Cervical right/14 mm | Roundish, ill-defined hilum hypoechogenic | Atypical lymphoid cytology | Biopsy: reactive follicular hyperplasia |
| Fernández-Prada et al.46 | 5 | 1st Pfizer Inc & BioN-Tech, 1–24 | Mean 44/F | NR | Supraclavicular left/NR | NR | Reactive; lymphocytic active germinal centres | NR |
| Kang and Kim22 | 1 | NR, Moderna, 14 | 59/M | Oral squamous carcinoma | Bilateral, cervical/NR | NR | Reactive; lymphocytic infiltrate | NR |
| Zeppa* | 1 | 2nd, Pfizer Inc & BioNTech, 14 | 58/F | Breast carcinoma | Cervical, right IB/12 mm | Oval, hypoechoic, hilum visible | Follicular hyperplasia, flow cytometry polyclonality | Clinical, negative |
| Yoshimoto et al.47 | 1 | NR | 70/F | Breast, colon carcinomas | Cervical/NR | Oval maintained US structure | Reactive; no metastasis | Clinical, resolution |
| Yu et al.35 | 1 | NR | 34/F | Negative | Axillary left/40 mm | Oval, hypoechoic, thickened cortex, hilum visible. | Reactive; granulocytes | Biopsy: reactive hyperplasia |
|
Ganga et al.52 |
1 | 2nd, Moderna, NR | 50/M | Arterial hypertension | Sub-mandibular left/50 mm | Roundish, hypoechoic | Reactive, inflammatory | Clinical, negative |
| Hagen et al.38 | 1 | 2nd, Moderna, 41 | 66/M | Lung carcinoma | Cervical level IV, supra-infra- or retro clavicular, axillary/10 to 24 mm | Ovoid to rounded shapes, partially hypo- and partially isoechogenic sharp borders, only partially detectable hilum | Follicular hyperplasia, no metastasis | Clinical, negative |
| 1 | 1st, Moderna, 76 | 41/F | Negative | Follicular hyperplasia, prominent germinal centre, tingible-body macrophages | Clinical, negative | |||
| 1 | 1st, Pfizer Inc & BioNTech, 22 | 47/F | Negative | Follicular hyperplasia, tingible-body macrophages. | Clinical, negative | |||
| 1 | 1st, Moderna, 63 | 47/F | NET appendix | Follicular hyperplasia, prominent germinal centre, tingible-body macrophages | Clinical, negative | |||
| 1 | 2nd, Pfizer Inc & BioNTech, 42 | 52/M | Lung carcinoma | Follicular hyperplasia, no metastasis | Clinical, negative |
- a Unpublished data, NR: not reported.
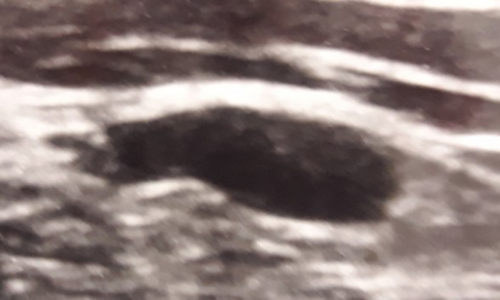
2.3 C19-LAP Cytological Features
Our research found 14 detailed reports describing the FNAC features of SLDI and C19-LAP.20-22, 35-38, 44-47, 52, 59, 72 From these 14 reports, FNAC findings for 25 cases of C19-LAP were retrieved; one unpublished case observed at our institution was also added, for a total of 26 cases. Aalberg et al.21 reported a case in the axillary lymph node of a patient suffering from stage IV renal cell carcinoma; FNAC smears were negative for neoplasia and showed reactive lymphoid cells. Trikannad et al.59 reported a case in the mediastinal lymph node of a patient suffering from melanoma. Cytological findings showed a reactive granulomatous process, negative for malignant cells. Gullotti et al.20 reported a case of metastasis for melanoma. Heaven et al.44 reported three cases; one showed polymorphous lymphoid cells suggestive of a reactive process; the second showed a reactive lymphoid population with scattered large, atypical cells of uncertain significance; in the third, they observed atypical lymphocytes suggestive of a lymphoproliferative disorder. In the last two cases a biopsy was performed, and in one case flow cytometry assessed a polyclonal reactive process. García-Molina et al.36 reported two cases and described similar cytological features, with a polymorphous cellularity, where numerous large cells with ovoid nuclei stood out, occupying almost the entire cytoplasm. Nuclei were sometimes bilobed or hyperchromatic, all of which exhibited nuclear hyperchromatism or large nucleoli. Numerous histiocytes with dense cytoplasm, sometimes polylobulated with prominent nucleoli, and a few histiocytes with granular bodies in their cytoplasm were also reported. Occasional apoptotic cells were also observed. All of this occurred in a lymphocytic background with lymphocytes with some atypia and irregular morphology, with nuclear notches and few plasma cells. Occasional neutrophil polymorphonuclear leukocytes and very scarce eosinophils could also be seen. These cytological findings were considered representative of nonspecific chronic adenitis. Dirven et al.72 reported one case in a patient suffering from a multiple endocrine neoplasia syndrome type 1 (MEN1), in which the FNAC of an axillary adenopathy revealed a benign reactive pattern without metastatic disease. Tan et al.37 described one case in which the smears showed a mixed lymphoid population with a range of small to large lymphocytes. An increased proportion of large, activated lymphocytes was also observed with a thin rim of bluish cytoplasm, and small, sometimes peripheral nucleoli. Germinal centre components, including lympho-histiocytic aggregates with follicular dendritic cells, tingible body macrophages and centroblasts, were also present. Tingible-body macrophages were particularly prominent, and many contained abundant karyorrhectic debris. Plasma cells and eosinophils were not prominent. No necrosis or granulomas were seen. The FNAC diagnosis was reactive process, suggestive of reactive follicular hyperplasia. Cardoso et al.45 reported one case of a roundish, 13.7 × 13.5 mm lymph node in which FNAC showed atypical lymphoid cytology; neither Reed-Sternberg cells nor malignant epithelial neoplastic cells were identified. The LN was removed and histological examination showed reactive follicular hyperplasia, with a concordant immunohistochemical evaluation. Fernández-Prada et al.46 reported five cases in which FNAC findings were described as merely showing reactive inflammatory signs, with lymphocytic infiltrate and active germinal centres. Kang & Kim22 reported one case in which FNAC of the left cervical lymph node revealed reactive LAP. Only small lymphoid cells were detected at the suspicious left lymph node. The authors of the present study (Caputo et al.) observed a case of cervical C19-LAP (unpublished). Smears showed polymorphous lymphoid cells ranging from small mature lymphocytes to large follicular centre cell populations (Figure 2), and macrophages with tingible bodies and capillary structures were present. Flow cytometry revealed balanced T and B cells; the latter were, in part, CD10-positive, with polyclonal light chain expression. Yoshimoto et al.47 reported one case of C19-LAP in the axillary lymph node in a patient suffering from breast carcinoma. FNAC excluded metastasis but cytological features were not provided. Yu et al.35 reported a case of C19-LAP in the axillary lymph node, for which cytological features were not detailed; however, clinical-cytological data favoured a suppurative adenitis, which was subsequently healed. Ganga et al.52 reported a large (50 mm) submandibular, hypoechoic lymph node with a cytological diagnosis of reactive inflammation, which shrank and disappeared after therapy. Hagen et al.38 reported five cases in which smears showed a florid reactive lymphadenopathy pattern, characterised by a mixed lymphoid population with lymphocytes at different stages of maturation, including many centroblasts admixed with numerous tingible body macrophages. Immunohistochemistry in one case revealed a reactive pattern with predominant CD3- and CD5-positive T cells, admixed with CD20-positive B cells without co-expression of CyclinD1, CD5, CD10 or CD23. Flow cytometry was performed in three cases; for two cases, the findings revealed T-cell predominant populations, with 72% CD3-positive T-cells vs 23% CD19-positive B cells, and 79% CD3-positive T cells vs 19% CD19-positive B cells; the other case showed a predominance of B-cells, with 55% CD19-positive B-cells vs 37% CD3-positive T cells. IgH rearrangement polymerase chain reaction testing revealed a polyclonal B-cell population, consistent with follicular hyperplasia. LN-FNAC diagnoses were histologically controlled in three cases and by clinical follow-up in the remaining 23 cases.
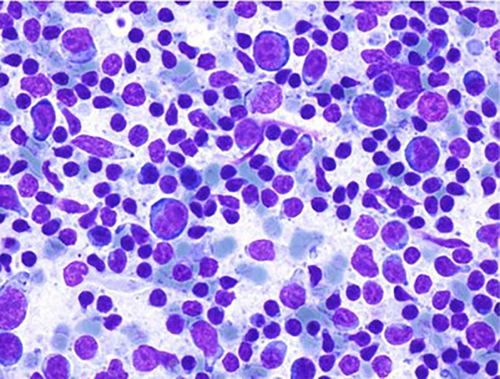
In summary, LN-FNAC of SLDI and C19 LAP cases has been performed in 9 patients during follow-up for different neoplasms, in 2 patients with comorbidity and 15 cases with negative clinical history. LN-FNAC diagnosed reactive hyperplasia in 22 cases and atypical or suspicious features in 2 cases: granulomatous process in one and metastasis in the other. Flow cytometry was performed in three cases to assess the polyclonality of the corresponding processes. Histological controls and follow-up confirmed the FNAC diagnoses. The clinical and cytological features of the reported cases are summarised in Table 2.
3 DISCUSSION
3.1 Post-vaccine lymphadenopathy
Post-vaccine lymphadenopathy due to reactive changes is a well-known phenomenon that may occur as a side effect of different vaccines, including bacillus Calmette-Guerin (BCG), hepatitis B, human papillomavirus, and tetanus vaccines,4-9, 12, 14 sometimes simulating a lymphoma,24, 57, 62 either clinically or pathologically. SARS-CoV-2 vaccines are the first mRNA vaccines to have been used on a large scale. Their mechanism of action differs from that of other vaccines, which are often based on attenuated or dead microorganisms, toxins, or bacterial surface proteins. SARS-CoV-2 vaccines are based on mRNA delivered into host cells, where it is translated into a protein, which is the target of the immune response.2, 15, 73, 74 Clinical trials have shown that the mRNA COVID-19 vaccines are highly immunogenic, and this quality might explain the higher rates of LAP reported as a side effect relative to other vaccines.26, 75, 76 The entity and the efficacy of the immune response to the same vaccine administration may depend on the constitutive and temporal differences in the reactivity between individuals. The actual incidence of C19-LAP is difficult to assess because of the heterogeneity of the sampled vaccinated populations, the lack of systematic investigations and selection bias. An additional limit to the evaluation of the incidence of SLDI and C19-LAP is that patients were controlled for pre-existing morbidities and for staging or follow-up of neoplastic diseases by 18F-FDG PET-CT, in which SLDI is more likely to be detected.24, 25, 47, 55, 66, 76 The morphological features of post-vaccine lymphadenopathies depend largely on the nature of the vaccine. Calmette-Guerin LAP has been reported as caseating granulomatous inflammation not dissimilar from tubercular lymphadenitis.77 Rubella LAP has been reported as lymphadenopathy with a prevalence of sinus histiocytosis.11 A case of tetanus LAP was suspected to be a T- cell lymphoproliferative disorder and was then diagnosed as vaccine-related “pseudolymphomatous” CD4 – cells, florid proliferation.14 All other LAPs (associated with measles, rubella, smallpox, cholera, typhus, tetanus, diphtheria, pertussis, salk (polio), influenza, and HPV) have been reported as diffuse, follicular, or combined diffuse and follicular lymph node hyperplasia with increased numbers of lymphoblasts, vascular and sinusoidal changes, and a variable number of eosinophils, plasma cells, and mast cells.11, 12 In addition to granulomatous or follicular hyperplasia, non-Covid-vaccine-related cases of lymphadenopathy caused by KFD,10 RDD and Langerhans cell histiocytosis,61 as well as post-transplantation like lymphadenopathy, have been reported.78
3.2 Pathology of COVID-19 post-vaccine lymphadenopathy
Lymph node excision and core-needle biopsy (CNB) were the most frequently performed procedures for the pathological evaluation of COVID-19 post-vaccine lymphadenopathy. We retrieved 48 cases of C19-LAP with histological control (excision or CNB)22, 23, 39, 43, 48, 49, 51, 53, 56, 57, 61, 62 and diagnosed as negative or reactive with or without follicular hyperplasia (37 cases). Two cases were reported as florid lymphoid hyperplasia54, 57; KFD was reported in four cases37, 39, 49, 56 (16.7%). Larkin et al.54 reported a focal increase in Epstein-Barr Virus (EBV)-positive cells, with other findings suggestive of prior infection. In one case, progressive transformation of germinal centres was also reported.54 Tintle et al.57 reported a case of Langerhans cell hyperplasia. Gogia et al.48 reported a case of RDD after COVID-19 infection. The pooled analysis of 38 cases showed a mean age of 40.2 ± 15.6 years old, 50.0% (18/36) of whom were female. Half of these patients had no prior medical history, while three patients (3/18, 16.7%) had a prior non-neoplastic medical history, including asthma, eczema, and hypothyroidism in the study by Tintle et al.57; steroid-dependent minimal-change renal disease was reported by Al Soub et al.56 and diabetes mellitus and hypertension by Tan et al.39 Cases reported by Ozutemiz et al.55 had a family history of breast cancer. Most cases of lymphadenopathy occurred on the same side as the vaccination site, with contra-laterality reported in two cases (2/18, 11.1%).79 The most common site of lymphadenopathy was the supraclavicular region (6/13, 46.2%), followed by the axillary (4/13, 30.8%) and cervical regions (3/13, 23.1%). The most commonly reported associated symptoms were fever and pain. The mean reported lymph node dimensions were 22.1 ± 14.7 mm. Ultrasound features were lymph node enlargement, cortical thickening, hypoechogenic areas, lost or partially detectable hilum and ill-defined borders.71
The cases of florid lymphoid hyperplasia and KFD had mean ages of 18.0 ± 5.0 years and 23.3 ± 7.5 years, respectively, which are significantly younger than those diagnosed with reactive changes or as negative for malignancy (44.2 ± 13.1 years old, p = 0.02). The mean length of the larger of the two lymph node dimensions did not differ significantly among these three diagnoses (reactive changes or negative for malignancy: 22.1 ± 18.2 mm, florid lymphoid hyperplasia: 15.5 ± 5.5 mm, KFD: 22.3 ± 7.1 mm). In the histopathological reports of C19-LAP, lymph nodes have mostly been described as florid follicular hyperplasia; a case of progressive transformation of germinal centres was also reported by Larkin et al.54 and a case of Langerhans cell hyperplasia57 was also reported. KFD has been reported in several studies.40-42, 64
3.3 Lymph node fine needle aspiration cytology
LN-FNAC is a useful and effective procedure in the diagnosis of lymphadenopathy.27-30, 32-34, 80, 81 The COVID-19 pandemic led to a worldwide reduction in the number of FNAC procedures performed, in general and in lymph nodes specifically. Paradoxically, the pandemic has indirectly enhanced and highlighted the role of LN-FNAC, which was the procedure of choice when more invasive core-needle or surgical biopsies were not available, particularly during the lockdowns, when access to hospitals was extremely restricted, even for therapeutic procedures.82 The role of LN-FNAC in C19-LAP can be also deduced by the evaluation of cytological and histological reports of C19-LAP; in a comprehensive review61 61 cases were collected from 25 different reports, including cases diagnosed by CNB, histology and LN-FNAC. In this review, 22 cases of C19-LAP were diagnosed by LN-FNAC and 39 by CNB or surgical excision, showing that LN-FNAC had a significant clinical role during the Covid pandemic. As reported above, in the cases analysed, in addition to reactive cases (22 cases) and malignant cases (1 case), “atypical lymphoid proliferation” was reported in 3 cases.44, 45 In one of these cases,44 LN-FNAC was repeated, and the case was diagnosed as reactive and confirmed on follow-up. In the remaining two cases, lymph nodes were excised and diagnosed as reactive on histology. The authors of the present study believe these cases might match the case described by Patil et al.62 of atypical follicular hyperplasia with light chain-restricted germinal centres after a COVID-19 booster. It is conceivable that the immunological hyperstimulation or the hyperreactivity caused by RNA vaccines in certain patients might be responsible for the marked hyperplasia of germinal centres and pseudo-clonality, as is the case in some cases of autoimmune diseases.83 It is worth noting that ancillary techniques were not performed on cytological material in any of these cases. It is not clear whether this was due to a shortage of diagnostic material or was merely the operators' choice; however, in these cases, ancillary techniques may be able to solve many of the diagnostic problems through proper phenotypisation and/or clonality assessment of the cell populations.28, 33, 80, 81, 84 Because LN-FNAC does not perform as well as the gold-standard histological evaluation in the identification of some specific lymphadenopathies and lymphomas,28 it has been broadly criticised.85, 86 This is unfair, in the authors’ opinion, because LN-FNAC is extremely valuable in excluding and sometimes confirming malignant processes, and it is particularly useful when CNB or histological excisions are hampered or difficult to perform, as was the case during the Covid lockdowns. In fact, as reported above, of all of the reported cases of C19-LAP diagnosed by LN-FNAC, 3 cases were confirmed by histology and 19 by follow-up, thus avoiding more complex and invasive procedures. Regarding the clinical background of C19-LAP cases diagnosed by LN-FNAC, a history of neoplasm was present in 8/26 cases (30%), and in the metastatic case the clinical history was negative. These findings confirm the crucial role of LN-FNAC in the management of oncological patients for the quick evaluation of new lymphadenopathies. Notably, Hagen et al.,38 who combined clinical data and cytological features and applied the proposed Sydney System,28 included C19-LAP in their cytological report in addition to excluding malignancy, thus enhancing the value of LN-FNAC.
4 CONCLUSIONS
LN-FNAC has a high diagnostic value in cases of SLDI and C19-LAP in patients with comorbidity or a history of neoplasia and in otherwise healthy people. The diagnosis of reactive processes and the exclusion of possible malignancies have been extremely useful in the context of the COVID-19 pandemic and particularly valuable when CNB or histological excisions were difficult to perform or were hampered, as was the case during the Covid lockdowns.
AUTHOR CONTRIBUTIONS
Drafting of manuscript: Alessandro Caputo, Valeria Ciliberti. Literature search: Alessia Caleo, Giuseppe Ciancia, Valeria Ciliberti. Study conception/design: Alessandro Caputo, Alessia Caleo, Pio Zeppa. Data acquisition: Alessandro Caputo, Immacolata Cozzolino, Valeria Ciliberti. Data analysis and interpretation: Alessandro Caputo, Pio Zeppa, Immacolata Cozzolino. Manuscript preparation: Alessandro Caputo, Pio Zeppa, Valeria Ciliberti. Manuscript review and approval: Alessandro Caputo, Pio Zeppa.
ACKNOWLEDGEMENT
Open Access Funding provided by Universita degli Studi di Salerno within the CRUI-CARE Agreement.
FUNDING INFORMATION
All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
All data supporting this article are available upon reasonable request to the corresponding author.