Interventional cytopathologist can successfully combine ultrasonographical and microscopic skills to narrow the differential diagnosis in fine needle aspiration of neck paraganglioma
Abstract
The interventional cytopathologist can successfully refine the differential diagnosis of a neck mass by combining ultrasonographical and morphological skills as shown in this case of head and neck paraganglioma, which was suspected thanks to ultrasound features and rapid-on site evaluation and further confirmed by immunocytochemistry on cell block and by the subsequent histologic evaluation.
1 CASE HISTORY
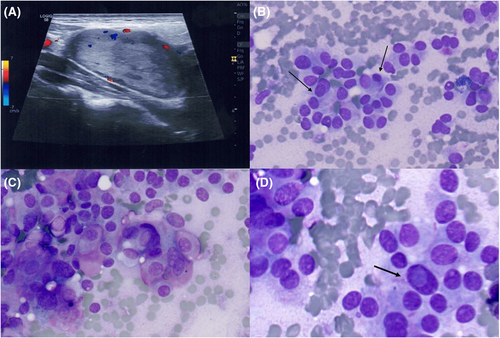
A 51-year-old man, upon noticing swelling in his neck, was referred for an ultrasound (US) in a community hospital; the scan hypothesised a parotid origin. Thus, fine needle aspiration (FNA) was performed at our academic institution by an interventional cytopathologist under US guidance. However, the US scan performed by the interventional cytopathologist failed to demonstrate an origin from the parotid gland (Figure 1A). Rapid on-site evaluation (ROSE) of the DiffQuik (DQ) stained smears revealed cells arranged in trabecular and solid groups (Figure 1B); in addition, a pseudo-follicular pattern was evident (Figure 1B). Additionally, DQ staining highlighted reddish metachromatic granules within the cytoplasm (Figure 1C); intranuclear cytoplasmic inclusions were also observed (Figure 1D). Based on these features, a preliminary differential diagnosis between a paraganglioma and a metastatic thyroid malignancy, either papillary or medullary thyroid carcinoma, was hypothesised. In view of the potential thyroid malignancy, additional ultrasound imaging of the thyroid gland was performed by the interventional cytopathologist. This revealed a normal thyroid gland, excluding a thyroid origin. An additional FNA pass was performed in order to obtain a cell block (CB) preparation. Haematoxylin and eosin-stained CB sections showed solid and trabecular cell groups (Figure 2A) with an evident plasmacytoid cytomorphology (Figure 2B), featuring a diffuse positivity for both chromogranin A and synaptophysin immunomarkers (Figure 2C). Moreover, the S100 immunostain revealed scattered spindle cells at the periphery of the cell groups (Figure 2D). Thus, a final diagnosis of paraganglioma was rendered. Upon this FNA diagnosis, magnetic resonance imaging scans were ordered, revealing a heterogeneous mass closely associated with the jugular-carotid vascular bundle and demonstrating discrete contrast enhancement; any connection with the parotid gland was further excluded.

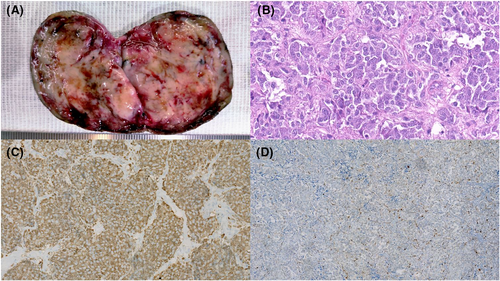
The subsequent surgical specimen showed a firm, rubbery, and well encapsulated 6 cm whitish mass featuring haemorrhagic areas (Figure 3A). The histology slides revealed an organoid-patterned neoplasm composed of polygonal cells featuring abundant eosinophilic and granular cytoplasm; the cells were arranged in nests or lobules partially surrounded by either elongated cells or fibro-vascular septa (Figure 3B). The main cells were diffusely positive for chromogranin A and synaptophysin (Figure 3C), whereas the immunohistochemical assay showed a positive S100 staining for the elongated cells (Figure 3D). These features confirmed the diagnosis of paraganglioma.
2 DISCUSSION
Paragangliomas are slow growing, usually benign, hypervascular tumours, traditionally classified into two subgroups based on anatomic location. The majority (90%) arise in adrenal paraganglia and are referred to as pheochromocytoma, while extra-adrenal paragangliomas are quite rare (10%). These latter can occur in virtually every anatomic site in which paraganglia can be found, although they are more frequent in the abdomen (85%), thorax (12%), and head and neck (H&N) region (3%). Carotid body tumour (CBT) is the most common paraganglioma in the H&N region.1
Usually, CBTs are asymptomatic masses, retrieved incidentally upon imaging procedures performed for other medical reasons, often misdiagnosed as metastatic lymph nodes and frequently referred for FNA. In recent years, the interventional cytopathologist, the proceduralist who performs both US guidance and FNA at the same time, has emerged as a new pathologist-clinician hybrid competent at both proper FNA sampling and the correct handling of aspirated material. Although still debated, the additional skills in performing US and interpreting the echographic features of the aspirated lesions can help the cytopathologist in narrowing the differential diagnoses.2 This approach may allow a better triaging of small tissue samples such as those obtained by FNA; in particular, by reviewing the US features, the cytopathologist can select a limited immunocytochemical panel sparing as much material as possible for further ancillary testing. Interventional cytopathology training is not standardised within cytopathology fellowship programs. However, several cytopathology fellowship programs include the acquisition of US skills among their goals. In this latter case, the young cytopathologists, besides the acquisition of the standard skills regarding both smearing techniques and ROSE, usually learn the practice of US during a radiology rotation. In Italy, the availability of cytopathologists with US skills is based on local traditions belonging to earlier efforts at performing FNA on palpable lesions, a practice progressively replaced by the acquisition of US-guidance skills by dedicated cytopathologists. In recent years, there has been worldwide renewed interest in this practice, confirmed by the increasing number of papers published about this topic.3, 4 Post-residency training programs focused on interventional pathology are also becoming available.5
As shown in the present case, the diagnostic process has benefited from both US and traditional microscopic skills. In fact, by excluding either a parotid or a thyroid origin, the interventional cytopathologist refined the differential diagnosis to CBT, thus performing on the CB a specific immunocytochemical panel. Besides the immunocytochemistry, the CB could highlight architectural features associated with CBT, such as the so-called zellballen (Figure 2A).
Usually, CBT US scans show a highly vascularised solid hypoechoic mass in the area of the carotid bifurcation usually associated with its splaying.6 The hypervascularisation may affect the smearing of the FNA due to cell obscuration by blood. However, in the present case, the mass did not show a marked vascularisation (Figure 1A). From a microscopic standpoint, the smears often show clusters of cells with granular eosinophilic cytoplasm and reddish metachromatic granules revealed by DQ. As in the present case, since the follicular arrangement of the tumour cells may suggest a metastasis from a thyroid carcinoma, a metastatic thyroid malignancy needs to be ruled out. The morphological, immunocytochemical and molecular features of the main differential diagnoses are summarised in Table 1.7-9
| Differential diagnosis | Cytomorphology | ICC | Molecular alterations |
|---|---|---|---|
| Papillary thyroid carcinoma | Oval-shaped nuclei, INCIs | TTF-1, PAX-8, Tg | BRAF V600E, RET/PTC |
| Medullary thyroid carcinoma | Reddish cytoplasmic granules, INCIs | Calcitonin, CEA | RET |
| Oncocytic thyroid tumour | Large, granular eosinophilic cytoplasm | TTF-1, PAX-8, Tg | RAS, mtDNA, CNV |
| Hyalinizing trabecular tumour | Elongated cells, INCIs, extracellular hyalin matrix | TTF-1, PAX-8, Tg, Ki67 (membrane staining) | PAX8-GLIS3 |
- Note: Here are highlighted the cytomorphological features which may overlap with those observed in paraganglioma.
- Abbreviations: CNV, copy number variations; INCI, intranuclear cytoplasmic inclusion; mtDNA, mitochondrial DNA; Tg, thyroglobulin.
To rule out these differential diagnoses, a combined approach including US scans of the thyroid gland and immunocytochemical staining of the CB can be useful to exclude a thyroid origin. In fact, CBTs are typically positive for neuroendocrine immunostains (chromogranin A, synaptophysin) while the sustentacular cells are positive for S100.8 Paragangliomas occur both in sporadic and familial forms; these latter are frequently associated with germline mutations in the succinate dehydrogenase subunits (SDHx), in particular in the SDHB, SDHC, and SDHD genes. The immunohistochemistry of SDHx subunits has been considered a useful tool for the prediction of SDHx mutations in paragangliomas.10 An additional rare occurrence is the thyroid paraganglioma; thyroid paragangliomas are extremely rare and are classified as inferior laryngeal paragangliomas. Usually, thyroid paragangliomas are non-functional and present as solitary thyroid nodules occasionally sampled by FNA. In these cases, an additional differential diagnosis, the hyalinizing trabecular tumour, should be taken into account (Table 1). In the present case, a thyroid paraganglioma was ruled out also by the US scans which showed a lack of relationship with the thyroid gland.
Unfortunately, FNA is unable to differentiate preoperatively between a benign and malignant CBT, though it can highlight nuclear atypia and an increased mitotic rate which are features associated with a malignant CBT.
3 CONCLUSION
CBT diagnosis on FNA is challenging. Thus, knowledge of the cytological and immunocytochemical characteristics of these tumours is advisable in the training of an interventional cytopathologist to cope with patients who need additional imaging evaluation in doubtful cases. Moreover, as shown by the present case, it is important for the interventional cytopathologist to acquire further skills in ultrasonography to narrow the differential diagnosis of neck swellings during the FNA procedure.
AUTHOR CONTRIBUTIONS
Conceptualisation: Anna Maria Carillo, Giancarlo Troncone, Claudio Bellevicine. Methodology: all authors: Investigation: all authors. Resources: all authors. Data curation: all authors. Writing—original draft preparation: Anna Maria Carillo, Claudio Bellevicine. Writing—review and editing: all authors. Visualisation: Anna Maria Carillo. Supervision: Giancarlo Troncone, Claudio Bellevicine.
ACKNOWLEDGEMENT
Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI-CARE Agreement. Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
CONFLICT OF INTEREST
Pasquale Pisapia has received personal fees as speaker bureau from Novartis, unrelated to the current work. Elena Vigliar has received personal fees (as consultant and/or speaker bureau) from Diaceutics and AstraZeneca, unrelated to the current work. Giancarlo Troncone has received personal fees (as speaker bureau or advisor) from Roche, MSD, Pfizer, Boehringer Ingelheim, Eli Lilly, BMS, GSK, Menarini, AstraZeneca, Amgen, and Bayer, unrelated to the current work. The other authors have nothing to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The clinical and imaging data as well as the morphological features of the case are fully described in this report. No further data are available.




