Nomogram to diagnosis of obstructive sleep apnoea-hypopnoea syndrome in high-risk Chinese adult patients
Jie Liu and Feng Pang are co-first authors and contributed equally to the work.
Funding information: No funding was received for conducting this study.
Abstract
Introduction
Many scales are designed to screen for obstructive sleep apnoea-hypopnoea syndrome (OSAHS); however, there is a lack of an efficiently and easily diagnostic tool, especially for Chinese. Therefore, we conduct a cross-sectional study in China to develop and validate an efficient and simple clinical diagnostic model to help screen patients at risk of OSAHS.
Methods
This study based on 782 high-risk patients (aged >18 years) admitted to the Sleep Medicine department of the Sixth Affiliated Hospital, Sun Yat-sen University from 2015 to 2021. Totally 34 potential predictors were evaluated. We divided all patients into training and validation dataset to develop diagnostic model. The univariable and multivariable logistic regression model were used to build model and nomogram was finally built.
Results
Among 602 high-risk patients with median age of 46 (37, 56) years, 23.26% were women. After selecting using the univariate logistic model, 15 factors were identified. We further used the stepwise method to build the final model with five factors: age, BMI, total bilirubin levels, high Berlin score, and symptom of morning dry mouth or mouth breathing. The AUC was 0.780 (0.711, 0.848), with sensitivity of 0.848 (0.811, 0.885), specificity of 0.629 (0.509, 0.749), accuracy of 0.816 (0.779, 0.853). The discrimination ability had been verified in the validation dataset. Finally, we established a nomogram model base on the above final model.
Conclusion
We developed and validated a predictive model with five easily acquire factors to diagnose OSAHS patient in high-risk population with well discriminant ability. Accordingly, we finally build the nomogram model.
1 INTRODUCTION
Obstructive sleep apnoea-hypopnoea syndrome (OSAHS) is the most common sleep-related breathing disorder, which is characterized by periodic, partial, or complete collapse of the upper airway during sleep, resulting in brief sleep arousals to restore airway patency.1 In the general population, the prevalence of OSAHS ranges from 9% to 38% and increases as age and body mass index, especially in males.2 A recent study found that obesity and OSAS adversely affect many organs and systems. Besides the factors affecting the diagnosis of the OSAS-obesity relationship, mutual organ interactions among the respiratory system, adipose tissue and intestines should not be ignored for prevention and treatment of OSAS and obesity.3 Untreated OSAHS is usually associated with adverse long-term health outcomes, such as systemic hypertension, pulmonary hypertension, heart failure, cardiac arrhythmias, diabetes mellitus, and stroke.4-6 OSAHS is also associated with poorer health-related quality of life and higher cost of healthcare resources.7-9 Recently, a special issue of Frontiers in Medicine for ‘Obstructive sleep apnoea syndrome (OSAS). What's new?’ also collected many recent researches about the OSAS, which is linked with a high risk of hypertension, cardiovascular diseases, daytime sleepiness, home and work-related accidents, and a consequent worsening of life quality. Numerous studies stress the importance of praecox diagnosis and a multidisciplinary approach in addressing all these situations.10
Currently, public awareness of OSAHS is increasing, and more and more high-risk patients are willing to evaluate the disease and seek treatment opportunities, but OSAHS remains severely underdiagnosed,11 so that a large proportion of patients do not receive any appropriate treatment, even though have no idea of this disease. At present, the gold standard for diagnosing OSAHS is polysomnography (PSG).12 However, the measurement of PSG requires the patient to stay overnight in the sleep monitoring centre. The PSG is time-consuming and expensive, additionally not all medical institutions have sleep monitoring centers and well-trained and professional sleep physicians. With the rising incidence of OSAHS due to rising obesity rates and an aging population, there is an urgent need to develop a simple and effective diagnostic tool that could allow for a wider screening and early diagnosis of OSAHS.
A lot of scales have been designed and developed to screen for OSAHS patients, including Espworth sleepiness scale (ESS), STOP-Bang and Berlin score. For example, a recent study found that a non-invasive assessment of low ArTH could help to identify an endotype with a lower predicted response to oral appliances in a clinical setting.13 However, previous studies shown that the specificity and negative predictive value (NPV) of these scales were not high, less than 60%,14 especially in the Chinese population.15 These scales cannot effectively discriminate the non-OSAHS patients and lead to a large proportion of unnecessary PSG screening. Therefore, we conduct a cross-sectional study in a Chinese population with high-risk to develop and validate a practical clinical model to screen patients of OSAHS.
2 MATERIALS AND METHODS
2.1 Patients
This is a cross-sectional study including all patients with suspected OSAHS admitted in respiratory care department of The Sixth Affiliated Hospital, Sun Yat-sen University from 2015 to 2021. The patients who were aged 18 years or older and suspected OSAHS patients admitted for polysomnography examination due to snoring or other related symptoms. The patients who were pregnant, had received OSAHS treatment in the past, or had mental illness and currently used the sedatives or antipsychotics, were excluded from this study. This study was approved by Ethics Committee of The Sixth Affiliated Hospital Sun Yat-sen University (2023ZSLYEC-001).
2.2 Diagnosis of OSAHS
The OSAHS was defined if apnoea-hypopnoea index (AHI) assessed by polysomnography was ≥5/h. After admission, all patients received polysomnography for an overnight monitoring. We invited two professional physicians with more than 5 years of experience to blindly and independently evaluate the monitoring data of polysomnography for each patient.
2.3 Selection and measurements of predictive factors
The potential predictors of OSAHS were included according to a systematic review of the current published literature in resent 10 years. We also invited several experts in this area to help us to evaluate and select the possible predictors for OSAHS. All these characteristics were collected at admission, which were abstracted and used as candidate predictors, including age, gender, OSAHS-related symptoms, ESS, blood pressure, BMI, lifestyle information, comorbidities, and circulating biochemical markers.
Finally, we selected the potential risk factors including the general factors (age, gender, and body mass index [BMI]), lifestyle factors (current smoking status and drinking status), the clinical syndrome of OSAHS (obstructive sleep apnoea, morning dry mouth and mouth breathing, heart palpitations at night, nocturia, and waking up dizzy or morning headaches), systolic and diastolic blood pressure, comorbidities (diabetes, hypertension, thyroid hypofunction, cardiovascular heart disease, and gastroesophageal reflux disease), ESS, AHI, and Berlin score as the potential predictors in this study. All above information was collected and recorded by study technicians or extracted from the electronic medical records after masking the confidential information of patients. Additionally, the circulating biochemical biomarkers, including fasting plasma glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, alkaline phosphatase, prothrombin, albumin, platelet count, and total bilirubin, were assessed in the central laboratory of the Sixth Affiliated Hospital, Sun Yat-sen University under the standard operation procedure. All data were double checked by the independent data reviewers to reconfirm the accuracy of the data; if any inconsistency we will discuss and finally confirm the data.
2.4 Statistical analyses
We divided all patients (n = 782) into two datasets according to the year of admission: the training dataset included the patients from 2015 to 2018 with 602 patients; while the validation dataset with 180 patients were from 2019 to 2021. The training dataset was used to establish the diagnosis model and the validation dataset was applied to validate the established model.
We calculated the mean (±standard deviation [SD]) or median (the 25th percentile and 75th percentile) for continuous variables and count (percentage %) for categorical variables, whenever appreciate. The student's t test for normally distributed data or Mann–Whitney U test for data with skewed distribution was used to compare the difference in characteristics between training and validation datasets or OSAHS or non-OSAHS patients in training dataset. Kolmogorov–Smirnov test was applied to evaluate the normal distribution of continuous variables. Binary or categorical variables were tested by using chi-square tests or Fisher's exact test.
Using the training dataset, we firstly evaluated and selected the individual associations between potential characteristics and OSAHS by using the univariate logistic regression model. The odds ratio (OR) and 95% confidential intervals (CIs) of different predictors were calculated. The statistically significant (P < 0.05) factors were selected and used for further selection by using multivariable logistic regression model. We used the stepwise selection method to help us select the predictor factors and finally built the diagnosis model for OSAHS. We built three best models for final selection. Based on the finally model, we established a nomogram model to rate the diagnosis value.
We used the receiver operator characteristic (ROC) curve and calculating the integrated area under the ROC curve (AUC) and its 95% CI to evaluate the discrimination of the diagnosis model. We also calculated the sensitivity, specificity, positive/negative predictive value, accuracy, positive/negative likelihood ratio (LR) and their 95% CIs to assess the discrimination of the diagnosis model. Use the DeLongs test, we compared the difference in AUC values between three different models and decided the final model.
Using the validation dataset, we verified the previously established diagnostic model, and evaluated the performance of the diagnostic models by checking the model discrimination and calibration. All above statistical values were estimated to reevaluate the performance of the diagnostic model.
All statistical analyses were applied by using the SAS, version 9.4 (SAS Institute, Inc. Cary, NC, USA). A two-sided P-value < 0.05 was considered as statistically significant.
3 RESULTS
A total of 782 patients aged 46 years (37–55 year) with high-risk of OSAHS were met the inclusion criteria and enrolled into this study (Table 1). Among them, 23.02% (n = 180) were women. Most of the patients' characteristics between training and validation datasets were comparable, only several laboratory tests, such as high-density lipoprotein, low-density lipoprotein, prothrombin, albumin, and platelet count, Berlin score, and age were slightly different between two groups of patients (all P-values > 0.05). Compared with non-OSAHS patients, those finally diagnosed OSAHS patients were more frequently to be men (79.24% vs. 64.36%), with older age (47 vs. 39.5 years), current drinkers (36.13% vs. 23.23%), with high blood pressures, with the symptom of morning dry mouth/mouth breathing and nocturia, higher AHI, ESS, and Berlin score, higher levels of fasting glucose, triglycerides, alanine aminotransferase, gamma-glutamyl transferase, and alkaline phosphatase; and lower levels of total bilirubin (all P-values < 0.05) (Table 2).
| Characteristics | Total (n = 782) | Training dataset (n = 602) | Validation dataset (n = 180) | P-value |
|---|---|---|---|---|
| Females | 180 (23.02) | 140 (23.26) | 40 (22.22) | 0.7726 |
| Age, years | 46 (37, 55) | 46 (37, 56) | 44 (35, 53) | 0.0147 |
| Current smokers | 275 (35.26) | 204 (34.00) | 71 (39.44) | 0.1799 |
| Current drinkers | 271 (34.70) | 209 (34.78) | 62 (34.44) | 0.9348 |
| BMI, kg/m2 | 26.72 ± 4.04 | 26.65 ± 4.1 | 26.94 ± 3.83 | 0.3896 |
| SBP, mmHg | 134.57 ± 14.19 | 134.36 ± 14.03 | 135.29 ± 14.74 | 0.4368 |
| DBP, mmHg | 87.69 ± 10.68 | 87.24 ± 10.28 | 89.17 ± 11.82 | 0.0498 |
| History of diabetes | 101 (12.93) | 85 (14.14) | 16 (8.89) | 0.0654 |
| History of hypertension | 240 (30.73) | 191 (31.78) | 49 (27.22) | 0.2449 |
| History of CHD | 63 (8.07) | 54 (8.99) | 9 (5.00) | 0.085 |
| History of thyroid hypofunction | 43 (5.51) | 29 (4.83) | 14 (7.78) | 0.1276 |
| History of GERD | 181 (23.18) | 135 (22.46) | 46 (25.56) | 0.3883 |
| Symptom | ||||
| Obstructive sleep apnoea | 363 (46.60) | 278 (46.41) | 85 (47.22) | 0.8482 |
| Morning dry mouth/mouth breathing | 708 (90.65) | 541 (90.02) | 167 (92.78) | 0.2643 |
| Heart palpitations at night | 161 (20.61) | 120 (19.97) | 41 (22.78) | 0.4135 |
| Nocturia | 311 (39.87) | 247 (41.17) | 64 (35.56) | 0.1775 |
| Waking up dizzy/morning headaches | 270 (34.66) | 214 (35.73) | 56 (31.11) | 0.2539 |
| AHI (times/h) | 24.6 (8.8, 56.9) | 25 (8.8, 56.3) | 23.35 (9.2, 58.95) | 0.7954 |
| ESS | 8 (5, 12) | 8 (5, 12) | 8 (5, 11) | 0.9633 |
| High Berlin score | 525 (71.43) | 382 (68.83) | 143 (79.44) | 0.0062 |
| Fasting plasma glucose, mmol/L | 5.21 (4.82, 5.94) | 5.23 (4.83, 5.96) | 5.18 (4.81, 5.83) | 0.2551 |
| Total cholesterol, mg/dL | 5.36 ± 1.18 | 5.37 ± 1.14 | 5.31 ± 1.31 | 0.5969 |
| HDL-cholesterol, mg/dL | 1.28 ± 0.38 | 1.32 ± 0.4 | 1.15 ± 0.25 | <0.0001 |
| LDL-cholesterol, mg/dL | 3.21 ± 0.87 | 3.13 ± 0.88 | 3.46 ± 0.78 | <0.0001 |
| Triglycerides, mg/dL | 1.76 (1.22, 2.64) | 1.76 (1.22, 2.59) | 1.71 (1.25, 2.76) | 0.9444 |
| AST, IU/L | 21.2 (17.73, 27.54) | 21.56 (17.77, 28) | 20.59 (17.62, 26.86) | 0.577 |
| ALT, IU/L | 27.52 (18.89, 42) | 27.99 (18.6, 41.47) | 26.79 (19.19, 45.23) | 0.8641 |
| GGT, IU/L | 33 (24, 53.33) | 33 (24, 53) | 34.07 (23.54, 53.55) | 0.9629 |
| ALP, IU/L | 79.25 ± 23.9 | 79.11 ± 23.57 | 79.74 ± 25.03 | 0.7718 |
| PT, s | 11.6 (11.12, 12.18) | 11.72 (11.27, 12.24) | 11.3 (10.8, 11.8) | <0.0001 |
| Albumin, g/dL | 43.51 (41.2, 46.02) | 43.81 (41.5, 46.36) | 42.42 (40.28, 45.25) | 0.0002 |
| Platelet count, 109/L | 231.99 ± 60.78 | 223.22 ± 57.18 | 261.54 ± 63.35 | <0.0001 |
| Total bilirubin, mg/dL | 12.87 ± 5.27 | 12.75 ± 5.28 | 13.21 ± 5.23 | 0.342 |
- Abbreviations: AHI, apnoea-hypopnoea index; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CHD, cardiovascular heart disease; DBP, diastolic blood pressure; ESS, Espworth sleepiness scale; GERD, gastroesophageal reflux disease; GGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PT, prothrombin; SBP, systolic blood pressure.
| Characteristics | Total (n = 602) | OSAHS (n = 501) | Non-OSAHS (n = 101) | P-value |
|---|---|---|---|---|
| Females | 140 (23.26) | 104 (20.76) | 36 (35.64) | 0.0012 |
| Age, years | 46 (37, 56) | 47 (39, 57) | 39.5 (32, 51) | <0.0001 |
| Current smokers | 209 (34.78) | 181 (36.06) | 28 (28.28) | 0.1378 |
| Current drinkers | 204 (34.00) | 181 (36.13) | 23 (23.23) | 0.0133 |
| BMI, kg/m2 | 26.65 ± 4.1 | 27.13 ± 4.01 | 24.2 ± 3.65 | <0.0001 |
| SBP, mmHg | 134.36 ± 14.03 | 135.44 ± 13.7 | 128.84 ± 14.44 | <0.0001 |
| DBP, mmHg | 87.24 ± 10.28 | 88.15 ± 10.15 | 82.67 ± 9.74 | <0.0001 |
| History of diabetes | 85 (14.14) | 76 (15.14) | 9 (9.09) | 0.1145 |
| History of hypertension | 191 (31.78) | 174 (34.66) | 17 (16.47) | 0.0006 |
| History of CHD | 54 (8.99) | 45 (8.96) | 9 (9.09) | 0.9678 |
| History of thyroid hypofunction | 29 (4.83) | 24 (4.78) | 5 (5.05) | 0.9089 |
| History of GERD | 135 (22.46) | 112 (22.31) | 23 (23.23) | 0.8409 |
| Symptom | ||||
| Obstructive sleep apnoea | 278 (46.41) | 239 (47.70) | 39 (39.80) | 0.1511 |
| Morning dry mouth/mouth breathing | 541 (90.02) | 461 (91.83) | 80 (80.81) | 0.0008 |
| Heart palpitations at night | 120 (19.97) | 99 (19.72) | 21 (21.21) | 0.7345 |
| Nocturia | 247 (41.17) | 216 (43.11) | 31 (31.31) | 0.0292 |
| Waking up dizzy/morning headaches | 214 (35.73) | 180 (36.00) | 34 (34.34) | 0.7533 |
| AHI (times/h) | 25 (8.8, 56.3) | 37.2 (15, 61) | 1.4 (0.7, 2.8) | <0.0001 |
| ESS | 8 (5, 12) | 8 (5, 12) | 7 (4, 10) | 0.0335 |
| High Berlin score | 382 (68.83) | 344 (75.27) | 38 (38.78) | <0.0001 |
| Fasting plasma glucose, mmol/L | 5.23 (4.83, 5.96) | 5.28 (4.87, 6.02) | 4.99 (4.75, 5.38) | 0.0006 |
| Total cholesterol, mg/dL | 5.37 ± 1.14 | 5.4 ± 1.13 | 5.18 ± 1.17 | 0.1389 |
| HDL-cholesterol, mg/dL | 1.32 ± 0.4 | 1.31 ± 0.4 | 1.4 ± 0.41 | 0.0798 |
| LDL-cholesterol, mg/dL | 3.13 ± 0.88 | 3.15 ± 0.88 | 3.03 ± 0.87 | 0.284 |
| Triglycerides, mg/dL | 1.76 (1.22, 2.59) | 1.79 (1.25, 2.64) | 1.5 (1.01, 2.35) | 0.0207 |
| AST, IU/L | 21.56 (17.77, 28) | 22 (17.97, 28) | 20.06 (17, 26.1) | 0.0775 |
| ALT, IU/L | 27.99 (18.6, 41.47) | 29 (19.64, 42.62) | 22.16 (16.48, 32) | 0.0013 |
| GGT, IU/L | 33 (24, 53) | 34.2 (25.07, 55.52) | 26 (19, 34.7) | <0.0001 |
| ALP, IU/L | 79.11 ± 23.57 | 79.98 ± 23.93 | 74.17 ± 20.88 | 0.046 |
| PT, s | 11.72 (11.27, 12.24) | 11.72 (11.27, 12.24) | 11.76 (11.31, 12.24) | 0.5306 |
| Albumin, g/dL | 43.81 (41.5, 46.36) | 43.67 (41.47, 46.2) | 44.3 (41.65, 47.67) | 0.1601 |
| Platelet count, 109/L | 223.22 ± 57.18 | 222.62 ± 56.87 | 226.28 ± 59 | 0.5689 |
| Total bilirubin, mg/dL | 12.75 ± 5.28 | 12.41 ± 4.62 | 14.78 ± 7.94 | 0.0208 |
- Abbreviations: AHI, apnoea-hypopnoea index; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CHD, cardiovascular heart disease; DBP, diastolic blood pressure; ESS, Espworth sleepiness scale; GERD, gastroesophageal reflux disease; GGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OSAHS, obstructive sleep apnoea-hypopnoea syndrome; PT, prothrombin; SBP, systolic blood pressure.
The univariate logistic regression model selected 15 factors from totally 34 factors were significantly related with OSAHS with all P-values < 0.05 (Table 3). Then, using the stepwise multivariate logistic regression model, we obtained the final model, including age (1.032 [95% CI: 1.010, 1.056], P = 0.005), BMI (1.221 [1.109, 1.345], P < 0.001), symptom of morning dry mouth/mouth breathing (3.033 [1.381, 6.663], P = 0.0057), Berlin score (2.154 [1.165, 3.983], P = 0.0144), and total bilirubin (0.939 [0.891, 0.989], P = 0.0179). Considering the previous evidence, we finally selected three models: Model 1: age, BMI, total bilirubin, symptom of morning dry mouth/mouth breathing, and Belin scores, Model 2: age, gender, BMI, total bilirubin, and symptom of morning dry mouth/mouth breathing, and Model 3: age, gender, BMI, total bilirubin, symptom of morning dry mouth/mouth breathing, and Berlin score.
| Characteristics | Univariate models | P-value | Multivariable model | P-value |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Females | 0.473 (0.298, 0.750) | 0.0015 | ||
| Age, years | 1.036 (1.018, 1.054) | <0.0001 | 1.032 (1.010, 1.056) | 0.005 |
| BMI, kg/m2 | 1.252 (1.169, 1.340) | <0.0001 | 1.221 (1.109, 1.345) | <0.0001 |
| Current smokers | 1.869 (1.133, 3.084) | 0.0144 | ||
| Current drinkers | 1.430 (0.890, 2.296) | 0.1393 | ||
| SBP, mm Hg | 1.037 (1.020, 1.055) | <0.0001 | ||
| DBP, mm Hg | 1.061 (1.035, 1.87) | <0.0001 | ||
| History of diabetes | 1.784 (0.862, 3.692) | 0.1188 | ||
| History of hypertension | 2.559 (1.471, 4.451) | 0.0009 | ||
| History of CHD | 0.930 (0.471, 1.835) | 0.8335 | ||
| History of thyroid hypofunction | 1.233 (0.509, 2.985) | 0.6421 | ||
| History of GERD | 0.949 (0.569, 1.583) | 0.8409 | ||
| Symptom | ||||
| Obstructive sleep apnoea | 1.380 (0.888, 2.144) | 0.1522 | ||
| Morning dry mouth/mouth breathing | 2.670 (1.475, 4.834) | 0.0012 | 3.033 (1.381, 6.663) | 0.0057 |
| Heart palpitations at night | 0.912 (0.537, 1.550) | 0.7345 | ||
| Nocturia | 1.662 (1.049, 2.634) | 0.0304 | ||
| Waking up dizzy/morning headaches | 1.075 (0.683, 1.692) | 0.7534 | ||
| AHI (times/h) | 67.03 (5.519, 814) | 0.001 | ||
| ESS | 1.047 (1.003, 1.093) | 0.0346 | ||
| High Berlin Score | 4.807 (3.039, 7.603) | <0.0001 | 2.154 (1.165, 3.983) | 0.0144 |
| Fasting plasma glucose, mmol/L | 1.129 (0.992, 1.286) | 0.0665 | ||
| Total cholesterol, mg/dL | 1.195 (0.944, 1.514) | 0.1394 | ||
| HDL-cholesterol, mg/dL | 0.611 (0.348, 1.072) | 0.0859 | ||
| LDL-cholesterol, mg/dL | 1.113 (0.863, 1.436) | 0.408 | ||
| Triglycerides, mg/dL | 0.994 (0.840, 1.175) | 0.9415 | ||
| AST, IU/L | 1.006 (0.992, 1.022) | 0.3956 | ||
| ALT, IU/L | 1.009 (0.998, 1.020) | 0.1027 | ||
| GGT, IU/L | 1.019 (1.007, 1.031) | 0.0014 | ||
| ALP, IU/L | 1.012 (1.000, 1.023) | 0.0455 | ||
| PT, s | 1.010 (0.974, 1.047) | 0.6043 | ||
| Albumin, g/dL | 0.997 (0.991, 1.004) | 0.4262 | ||
| Platelet count, 109/L | 0.999 (0.995, 1.003) | 0.5683 | ||
| Total bilirubin, mg/dL | 0.933 (0.893, 0.975) | 0.0019 | 0.939 (0.891, 0.989) | 0.0179 |
- Abbreviations: AHI, apnoea-hypopnoea index; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CHD, cardiovascular heart disease; DBP, diastolic blood pressure; ESS, Espworth sleepiness scale; GERD, gastroesophageal reflux disease; GGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OSAHS, obstructive sleep apnoea-hypopnoea syndrome; PT, prothrombin; SBP, systolic blood pressure.
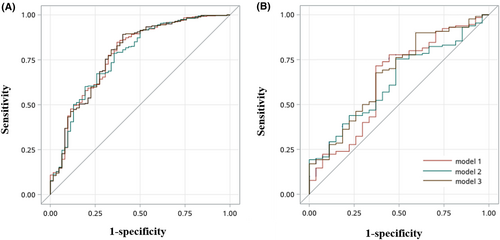
In both training dataset and validation dataset, the above three models presented similar discriminant ability; however, the best one is Model 1 with an AUC of 0.780 (0.711, 0.848), sensitivity of 0.848 (0.811, 0.885), specificity of 0.629 (0.509, 0.749), accuracy of 0.816 (0.779, 0.853), positive predictive value of 0.930 (0.903, 0.958), negative predictive value of 0.415 (0.315, 0.515), positive likelihood ratio of 2.286 (1.538, 3.034), and negative likelihood ratio of 0.242 (0.167, 0.316) in training dataset (Table 4). Even though, compared with other two models, the ROC curve and AUC were not statistically significant with P-value = 0.5433 for Model 2 versus Model 1 and P-value = 0.9518 for Model 3 versus Model 1 in training dataset (Figure 1). Similarly, in validation dataset, the comparisons were also non-significant (all P-value > 0.05).
| Models | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Training dataset | ||||||||
| Model 1 | 0.780 (0.711, 0.848) | 0.848 (0.811, 0.885) | 0.629 (0.509, 0.749) | 0.816 (0.779, 0.853) | 0.930 (0.903, 0.958) | 0.415 (0.315, 0.515) | 2.286 (1.538, 3.034) | 0.242 (0.167, 0.316) |
| Model 2 | 0.770 (0.701, 0.839) | 0.768 (0.726, 0.809) | 0.646 (0.530, 0.762) | 0.751 (0.711, 0.790) | 0.930 (0.902, 0.957) | 0.313 (0.235, 0.392) | 2.170 (1.447, 2.892) | 0.360 (0.268, 0.451) |
| Model 3 | 0.780 (0.711, 0.849) | 0.889 (0.857, 0.922) | 0.597 (0.475, 0.719) | 0.846 (0.812, 0.881) | 0.928 (0.901, 0.955) | 0.481 (0.369, 0.592) | 2.205 (1.533, 2.878) | 0.186 (0.119, 0.252) |
| Validation dataset | ||||||||
| Model 1 | 0.637 (0.512, 0.762) | 0.862 (0.802, 0.921) | 0.296 (0.124, 0.469) | 0.764 (0.698, 0.831) | 0.855 (0.795, 0.915) | 0.308 (0.130, 0.485) | 1.224 (0.913, 1.536) | 0.467 (0.130, 0.805) |
| Model 2 | 0.627 (0.518, 0.737) | 0.746 (0.671, 0.821) | 0.556 (0.368, 0.743) | 0.713 (0.643, 0.784) | 0.890 (0.831, 0.949) | 0.313 (0.181, 0.444) | 1.679 (0.951, 2.407) | 0.457 (0.252, 0.662) |
| Model 3 | 0.668 (0.552, 0.783) | 0.576 (0.407, 0.744) | 0.065 (0.021, 0.108) | 0.172 (0.113, 0.231) | 0.141 (0.082, 0.199) | 0.364 (0.163, 0.565) | 0.616 (0.433, 0.798) | 6.576 (1.452, 11.70) |
- Note: Model 1: adjusted for age, BMI, TB, syndrome of morning dry mouth or mouth breathing and high Berlin score. Model 2: adjusted for age, gender, BMI, TB, and syndrome of morning dry mouth or mouth breathing. Model 3: adjusted for age, gender, BMI, TB, syndrome of morning dry mouth or mouth breathing and high Berlin score. Training dataset: Model 2 versus Model 1: P-value = 0.5433, Model 3 versus Model 1: P-value = 0.9518. Validation dataset: Model 2 versus Model 1: P-value = 0.7778, Model 3 versus Model 1: P-value = 0.1171.
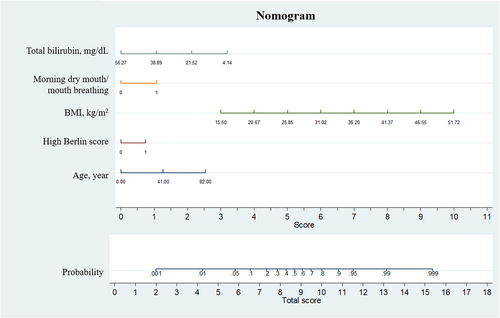
We finally built the nomogram based on the Model 2 (Figure 2). The points assigned to each factor was on the score line and the total probability and score were on the bottom. For example, a patient aged 40 years with a BMI of 25 kg/m2, high Berlin score, with total bilirubin of 21.52 mg/dL, with a symptom of mouth breathing has an approximately 80% chance to be OSAHS.
4 DISCUSSION
Using the cross-sectional data of 782 patients, we developed and validated a practical nomogram model to help us screen the OSAHS patient in a high-risk Chinese population with a well discriminant ability. Our nomogram included five factors provides an efficient, convenient, and simple tool for clinical diagnosis of OSAHS.
Previous evidence suggested that the males have a high risk of OSA, the rate of males versus females in OSA patient populations was 8:1.16 However, in our study, after stepwise multivariable selection, the gender was not included into the final diagnostic model. The potential reason may be that the patients in our study were those high-risk patients of OSAHS. Although the males were more likely to develop OSA than females, among those high-risk patients, no matter males or females develop OSA in the similar risk. Additionally, in undiagnosed OSA populations, the ratio of males versus females was 2:1, which indicated that women with OSA are less likely to be evaluated and diagnosed.6 Moreover, evidence suggested that OSA in women may be diagnosed late in the course of the disease or may not be aggressively treated.6 Considering the gender difference in OSAHS, we additionally included gender into other models, but non-significant differences from our final model (without gender) were detected in both training and validation datasets.
Age17-19 and BMI16, 20 have been confirmed as the critical risk factors of OSAHS, and all these two factors were included in our final model as a diagnostic factor. Our nomogram model indicated that BMI indicated a high contribution to OSAHS diagnosis. Previous evidence not only suggested that BMI increase results in OSAHS, nearly a 10% increase in weight was associated with a sixfold greater risk of developing OSA among persons initially free of OSA,20 but also the weight loss according to surgery or diet are significantly decrease in the severity of OSAHS.16 Besides that, the OSA patients with obesity would have a high risk of cardiovascular diseases in further.21 The underlying mechanism of obesity and OSAHS might be caused by the effects of intermittent hypoxia on adipose tissue.22, 23 Also, insufficient sleep may counteract with dysmetabolic changes.24 Moreover, both OSAHA and obesity induced the systemic pro-inflammatory changes and finally enhanced the risk of cardiovascular diseases.25, 26 Older age adults (age ≥65) have a twofold to threefold higher prevalence of OSA than those aged less than 65 years.17-19 However, the under mechanism of the influence of age on OSAHS is still uncertain.
Bilirubin, an endogenous antioxidant in human vascular endothelial cells, provides an additional mechanism for hypoxic adaptation mediated through feedback inhibition of the heme biosynthetic pathway.27, 28 A meta-analysis of 11 studies found an inverse relationship between bilirubin concentrations and atherosclerosis severity in men with a 6.5% decrease in cardiovascular events for every 1 mol/L increase in bilirubin.29 Among patients with OSAHS, the hyoxaemia would introduce a series of chain reactions in metabolism and changes in metabolism. Thus, the metabolism biomarkers might be the sensitivity response factors of OSAHS. This hypothesis has been supported by previous evidence,30 which found an increase in bilirubin levels of patients with OSA in the morning. Consistently, after the competition of multiple variables, total bilirubin levels were selected as a critical indicator for OSAHS in Chinese adults.
Morning dry mouth or mouth breathing also has been considered as an screening factors for sleep apnoea, because the dry mouth symptom significantly correlated with snoring and sleep apnoea.31 The specificity and diagnostic accuracy of the STOP-Bang questionnaire have been improved by integrating dry mouth. Besides of other influence factors, such as hypertension and diabetes, due to the open-mouth breathing during night sleep, the patients with OSA more frequently report a more typical dry mouth symptom in the morning. Our study reconfirmed that the morning dry mouth or mouth breathing might be a valuable and typical predictor for OSA.32
Using the data from a cross-sectional study in Chinese adults, we develop a convenient, simple, and efficient diagnostic nomogram model, which could efficiently help clinicians to quickly discriminant the OSAH patients from those high-risk population. All the risk factors were easily obtained, which might be have a wider applicability in practice. However, this study has several limitations. This is single centre study, which included the patients from one hospital and might include selection bias. Further need an external validation in other population, although we validated the model efficient and utility in a special training population form the same centre. We included many risk factors; however, there might be other diagnostic factors still not included, for example other saliva biomarkers33 and gene assessments.34, 35
Our study provided an efficient, convenient, and simple nomogram scale with five factors to help clinical screening OSAHS patients in those high-risk population in China, which has a wider applicability in practice. However, due to the limits of cross-sectional design and single centre study, the utility and feasibility need further reconfirmed in other population and verified by prospective studies.
AUTHOR CONTRIBUTIONS
Jie Liu contributed to conceptualization. Feng Pang contributed to methodology. Xiaofeng Huang and Minmin Lin contributed to data collection and supervision. Xiangmin Zhang and Tianrun Liu contributed to project administration. Wenmin Deng contributed to data curation. Zhen Long contributed to reviewing and editing of the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethics Committee of The Sixth Affiliated Hospital Sun Yat-sen University (Approval number: 2023ZSLYEC-001). Consent to participate is waived due to it was an observational study.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.




