Pneumocystis jirovecii Pneumonia in Cancer Patients, a Lethal Yet Fully Preventable Disease: Insights From a Tertiary Cancer Center in East India
S. Chatterji and V. Franchi contributed equally to this study.
ABSTRACT
Background
Pneumocystis jirovecii pneumonia (PCP) is an unrecognized infection in non-HIV patients, particularly those with solid and hematologic malignancies. These patients experience higher mortality rates. This study aims to describe the incidence, initial characteristics, management, and outcomes of PCP at a tertiary cancer care center.
Methods
This retrospective observational study included all patients who underwent P. jirovecii PCR testing at our center from January 2019 to January 2022. PCP was diagnosed in PCR-positive patients. Data on demographics, treatment, and outcomes were extracted from medical records. The primary outcomes were ICU admission and 21-day mortality. Statistical analysis compared PCR-positive and PCR-negative patients, with a specific focus on lung cancer patients, and analyzed determinants of 21-day mortality in PCP patients.
Results
Of the 345 patients suspected of PCP, 54 (15.7%) were diagnosed with PCP. PCP patients were generally older. None of the PCP patients were on prophylaxis, compared to 14.8% of PCR-negative patients. In lung cancer patients, age and radiotherapy within the past year were significantly associated with a PCP diagnosis. The 21-day mortality rate among PCP patients was 35.4%. Independent risk factors for mortality included age and hematologic malignancy, while recent chemotherapy and higher neutrophil counts were associated with lower mortality.
Conclusion
PCP is associated with the highest mortality in patients with hematologic malignancies and lung cancer. The findings underscore the importance and efficacy of prophylaxis in at-risk groups and should raise awareness for the diagnosis of PCP in overlooked populations, such as older cancer patients and those undergoing radiotherapy.
1 Introduction
Pneumocystis jirovecii pneumonia (PCP) is a well-known opportunistic infection in HIV patients. However, this infection appears to be overlooked in non-HIV patients, such as those with solid and hematological malignancies. Available data show that PCP is associated with higher mortality in non-HIV patients than in HIV patients [1]. Several risk factors have been described, such as age, lung cancer, current chemotherapy, chest or brain radiotherapy, use of prolonged high dose steroid therapy, lymphopenia, and low CD4 lymphocyte count [2]. Prophylaxis with trimethoprim/sulfamethoxazole is highly effective, as evidenced by studies [3, 4]. Recently, guidelines have recommended prophylaxis for high-risk hematologic malignancy patients, including those undergoing hematopoietic stem cell transplantation (HSCT), those with acute lymphoblastic leukemia (ALL), and those receiving a combination of fludarabine, cyclophosphamide, and rituximab [5, 6]. Interestingly, pediatric guidelines recommend prophylaxis for all children with solid cancer and receiving treatment known to cause lymphopenia, and in those with pre-existing lung disease receiving chemotherapy [7]. A number of guidelines for both solid and hematological malignancies concur on the necessity for prophylaxis in patients undergoing high-dose (≥ 20 mg/day) prolonged steroid therapy (≥ 2–4 weeks, according to guideline) [6, 8, 9]. The first-line treatment for PCP is trimethoprim/sulfamethoxazole [10]. However, the role of corticosteroid adjuvant therapy (CAT) in PCP in non-HIV patients is still controversial [11-13].
We aimed to describe the incidence of PCP among PCP suspected cancer patients, and the initial characteristics, management, and outcomes of PCP patients in a tertiary care center treating patients with solid and hematologic malignancies.
2 Methods
2.1 Ethical Statement
Our study protocol was approved by Tata Medical Center Institutional Review Board, under number: EC/WV/TMC/30/24.
2.2 Study Design, Definitions, and Included Population
Patients were selected if they had undergone P. jirovecii DNA detection in respiratory specimens by quantitative real-time PCR from January 2019 to January 2022. The EORTC/MSGERC definition criteria for proven PCP requires the presence of clinical and radiological criteria with direct examination or immunofluorescence staining demonstrating the presence of P. jirovecii in tissue or respiratory specimens [9]. Probable PCP is defined as the presence of host factors, clinical and radiological criteria, and positive PCR from respiratory specimen or positive of Beta-D-Glucan in serum. Since PCP–PCR is only performed on individuals clinically and radiologically suspected of having PCP at our center, and since all patients are cancer patients meeting the host factor criteria, all patients with positive PCR were classified as probable PCP in this study. Data on demographics, biological results, and outcomes were collected from electronic medical records for both PCR-positive and PCR-negative patients. More detailed data regarding treatment received and disease stage were collected from the patients diagnosed with lung cancer. Data on radiological findings and PCP treatment were collected for PCR-positive patients only.
Patients were considered to have recently received chemotherapy if the patient had received chemotherapy within 1 month before the PCP diagnosis. Previous exposure to steroid therapy was considered if patients had been receiving steroids for at least 3 weeks at a minimum dose of 20 milligrams per day within 1 month of PCP diagnosis.
The predefined outcomes included intensive care unit (ICU) admission and 21-day mortality.
Of note, the local protocol of our center recommends PCP prophylaxis for patients with ALL, patients post HSCT, and for those with brain tumor receiving temozolomid and/or high-dose steroids.
2.3 Microbiological Diagnosis
2.3.1 Respiratory Samples Used
Pneumocystis PCR was performed on respiratory samples only. These were either sputum, endotracheal secretion, or bronchoalveolar lavage samples.
2.3.2 DNA Extractions From Respiratory Samples
A total of 200 µL of the samples were used for DNA extractions, which were carried out with a QIAamp DNA mini kit (Qiagen, Hilden, Germany). Samples were previously dissolved v/v in 200 µL of AL buffer, mixed with 20 µL of proteinase K 20 mg/mL (Qiagen, Hilden, Germany) at 56°C until complete tissue digestion. The remaining steps were performed according to the manufacturer's kit protocol. DNA samples concentration and purity were estimated by UV spectrophotometry (Nanodrop 1000, Thermo Scientific, USA) at 260 and 280 nm.
2.3.3 PCR Characteristics
The PCR protocol for the PneumoGenius multiplex kit (PathoNostics, the Netherlands) was performed on the AriaMx PCR System (Agilent, USA) according to the manufacturer's instructions.
2.4 Statistical Analysis
Analysis were performed in the total population comparing PCR-positive patients and PCR-negative patients. The same analysis comparing PCR-positive and PCR-negative patients was performed in the subset of lung cancer patients on the variables collected specifically in this subgroup. In the subset of patients diagnosed with PCP, analyses were performed to search for variables associated with the following outcomes: mortality at Day 21 and ICU admission.
Studied variables were described as percentages for dichotomous variables and as medians with interquartile range (IQR) for continuous variables. In percentage calculation, the number of missing values was excluded from the denominator. Nonparametric tests were used to compare groups (Fisher exact, Mann–Whitney U, chi-square, and Spearman tests), as appropriate. Kaplan–Meier curves were compared between groups using the log-rank (Mantel–Cox) test. Determinants of PCR positivity in the total cohort and in the lung cancer patients subset, and determinants for mortality at Day 21 in the PCR-positive subgroup, were assessed using stepwise binary logistic regression, and expressed as odd ratios (ORs) with their 95% confidence intervals (95% CI). In the subset of PCP diagnosed patients, noninteracting variables with medical meaning and p values obtained in univariate analysis < 0.15 were included in a logistic regression multivariate model for mortality at Day 21. Similar logistic regression multivariate analysis was performed in the lung cancer patients' subset, searching for variables associated with PCR positivity. A p value < 0.05 was considered significant.
All analyses were performed using R Statistical Software (v4.4.1; R Core Team 2024).
3 Results
3.1 Included Population
A total of 345 patients, suspected of PCP, were included (225 males [65.2%], median age 60.1 [IQR 49.9, 66.8] years) over the study period. Among patients suspected of PCP, 54 (15.7%) had a positive P. jirovecii PCR and were thus diagnosed with PCP. Over the same period, a total of 136,278 individual patients attended our hospital as inpatients or outpatients. Thus, 1.2 out of 1000 patients every year are suspected of PCP, and the incidence of PCP in our hospital is calculated 0.19 out of 1000 patients per year. The background disease was hematological malignancy in 148 (42.9%) patients and solid cancer in 189 (54.8%), with 87 (25.2%) patients presenting with lung cancer. Rest eight patients classified as nonmalignant hematological diseases suffered from myelodysplastic syndrome, myeloproliferative syndrome, aplastic anemia, and inherited bone marrow disease. More detailed data regarding background disease are available in Table 1. Five (2.0%) patients had positive HIV serology; all had good disease control. A total of 138 (40.0%) patients were admitted in the ICU. Mortality at 21 days occurred in 97 (31.1%) patients.
| Descriptive analysis | Univariate analysis | |||||
|---|---|---|---|---|---|---|
| All patients | PCR negative | PCR positive | ||||
| n | 345 | 291 | 54 | p value | OR (95% CI) | p value |
| Demographics | ||||||
| Age (years) | 60.11 [49.90, 66.82] | 59.32 [49.36, 66.22] | 63.22 [56.01, 70.38] | 0.010 | 1.024 (1.005–1.047) | 0.023 |
| Sex (male) | 225 (65.2) | 185 (63.6) | 40 (74.1) | 0.183 | 1.637 (0.87–3.244) | 0.139 |
| Background disease | ||||||
| Hematological malignancy | 148 (42.9) | 126 (43.3) | 22 (40.7) | 0.842 | 0.900 (0.500–1.620) | 0.727 |
| AML | 31 (9.0) | 29 (10.0) | 2 (3.7) | 0.223 | 0.347 (0.055–1.203) | 0.157 |
| ALL | 33 (9.6) | 29 (10.0) | 4 (7.4) | 0.738 | 0.723 (0.208–1.937) | 0.559 |
| NHL | 50 (14.5) | 42 (14.4) | 8 (14.8) | 1.000 | 1.031 (0.426–2.236) | 0.942 |
| HL | 8 (2.3) | 5 (1.7) | 3 (5.6) | 0.219 | 3.365 (0.674–14.146) | 0.104 |
| MM | 27 (7.8) | 22 (7.6) | 5 (9.3) | 0.880 | 1.248 (0.403–3.216) | 0.670 |
| NMHD | 8 (2.3) | 6 (2.1) | 2 (3.7) | 0.807 | 1.827 (0.263–8.18) | 0.468 |
| Solid cancer | 189 (54.8) | 159 (54.6) | 30 (55.6) | 1.000 | 1.038 (0.58–1.874) | 0.901 |
| Lung cancer | 87 (25.2) | 73 (25.1) | 14 (25.9) | 1.000 | 1.045 (0.523–1.99) | 0.896 |
| Other solid cancer | 102 (29.6) | 86 (29.6) | 16 (29.6) | 1.000 | 1.004 (0.519–1.867) | 0.991 |
| Lung metastasis | 38 (11.0) | 32 (11.0) | 6 (11.1) | 1.000 | 1.012 (0.366–2.397) | 0.980 |
| Positive HIV serology | 5 (2.0) | 3 (1.5) | 2 (4.3) | 0.509 | 3 (0.387–18.621) | 0.236 |
| Treatments previously received | ||||||
| HSCT | 17 (4.9) | 14 (4.8) | 3 (5.6) | 1.000 | 1.164 (0.262–3.723) | 0.817 |
| Chemotherapy within 1 month | 194 (56.2) | 168 (57.7) | 26 (48.1) | 0.248 | 0.68 (0.378–1.218) | 0.194 |
| High dose steroids exposure | 11 (3.2) | 9 (3.1) | 2 (3.7) | 1.000 | 1.197 (0.179–4.809) | 0.822 |
| PCP prophylaxis | 40 (12.3) | 40 (14.8) | 0 (0.0) | 0.006 | ||
| Biological features at baseline | ||||||
| LDH (U/L) | 416.00 [260.00, 789.00] | 434.50 [258.00, 838.25] | 404.00 [275.00, 596.00] | 0.634 | 1 (1–1) | 0.919 |
| Procalcitonin (ng/mL) | 0.48 [0.22, 2.84] | 0.54 [0.21, 3.84] | 0.46 [0.27, 1.18] | 0.678 | 0.991 (0.952–1.017) | 0.591 |
| Lymphocytes (103/µL) | 0.60 [0.30, 1.10] | 0.50 [0.20, 1.10] | 0.70 [0.40, 1.10] | 0.166 | 0.979 (0.765–1.112) | 0.801 |
| ANC (103/µL) | 5.80 [2.30, 9.83] | 5.55 [2.10, 9.60] | 6.55 [3.92, 10.75] | 0.068 | 1.020 (0.98–1.058) | 0.312 |
| WBC (103/µL) | 7.60 [3.70, 11.95] | 7.35 [3.32, 11.45] | 9.30 [5.50, 13.17] | 0.048 | 1.016 (0.989–1.04) | 0.217 |
| Sample used for Pneumocystis PCR | ||||||
| Sputum | 159 (46.1) | 131 (45.0) | 28 (51.9) | 0.437 | 1.315 (0.735–2.363) | 0.356 |
| ETS | 67 (19.4) | 56 (19.2) | 11 (20.4) | 0.996 | 1.074 (0.499–2.15) | 0.848 |
| BAL | 119 (34.5) | 104 (35.7) | 15 (27.8) | 0.330 | 0.692 (0.354–1.29) | 0.260 |
| Outcomes | ||||||
| ICU admission | 138 (40.0) | 115 (39.5) | 23 (42.6) | 0.785 | 1.135 (0.625–2.039) | 0.672 |
| ICU LOS (days) | 0.00 [0.00, 6.00] | 0.00 [0.00, 6.00] | 0.00 [0.00, 5.50] | 0.846 | 0.995 (0.952–1.032) | 0.814 |
| In ICU death | 55 (15.9) | 43 (14.8) | 12 (22.2) | 0.242 | 1.648 (0.776–3.304) | 0.173 |
| In hospital LOS (days) | 10.00 [5.00, 18.00] | 10.00 [5.00, 18.00] | 11.00 [7.00, 16.00] | 0.406 | 1.007 (0.985–1.026) | 0.512 |
| In hospital death | 110 (32.8) | 89 (31.6) | 21 (39.6) | 0.323 | 1 (1–1) | 0.709 |
| Lost to follow-up before Day 21 | 33 (9.6) | 27 (9.3) | 6 (11.1) | 0.866 | 1.222 (0.438–2.939) | 0.674 |
| Mortality Day 21 | 97 (31.1) | 80 (30.3) | 17 (35.4) | 0.593 | 1.261 (0.649–2.385) | 0.482 |
- Abbreviations: ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; ANC, absolute neutrophil count; BAL, bronchoalveolar lavage; ETS, endotracheal secretion; HIV, human immunodeficiency virus; HL, Hodgkin lymphoma; HRCT, high-resolution chest tomography; HSCT, hematopoietic stem cells transplant; ICU, intensive care unit; LDH, lactate dehydrogenase; LOS, length of stay; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; NMHD, nonmalignant hematological disease; PCP, Pneumocystis jirovecii pneumonia, WBC: white blood count.
3.2 Comparison of PCR-Positive and PCR-Negative Patients, in the Total Cohort and in the Subgroup of Lung Cancer Patients
Comparison between patients diagnosed with PCP and patients suspected but not confirmed shows that PCP patients were significantly older (OR, 1.024; 95% CI, 1.005–1.047; p = 0.023). None of the PCP patients was receiving PCP prophylaxis, whereas 40 (14.8%) PCP-negative patients were under prophylactic therapy (p = 0.006). All patients receiving prophylaxis had a background of hematological malignancy. Among hematological malignancy patients not receiving prophylaxis, 20.2% (22/109) were diagnosed with PCP. No significant difference was noted between PCP-positive and PCP-negative patients regarding the type of underlying disease, the biological features at baseline, or regarding outcomes such as ICU admission and 21-day mortality (see Table 1). Survival analysis comparing PCP-positive and PCP-negative patients showed no significant difference, see Figure S1.
We analyzed the lung cancer patients separately, focusing on possible PCP risk factors and especially cancer stage and received treatments. In this subgroup also, older age was associated with more PCP diagnoses (OR, 1.154; 95% CI, 1.051–1.289; p = 0.005). Stage 3 lung cancer was associated with positivity of P. jirovecii PCR (OR, 3.812; 95% CI 1.119–12.994; p = 0.037). History of lung radiotherapy within 1 year was strongly associated with PCP diagnosis (OR 8.308; 95% CI 2.471–31.128; p = 0.001). Cancer treatment with chemotherapy or other agents such as tyrosine kinase inhibitors (TKI) and check-point inhibitors (CPI), was not associated with a greater risk of PCP. None of the lung cancer patient had received high dose steroids for at least 1 month, none of them received PCP prophylaxis. Among lung cancer patients suspected with PCP, PCP diagnosis was not associated with different outcomes regarding ICU admission, hospital length of stay, and mortality at Day 21. See Table S1.
In multivariate analysis, age and radiotherapy within 1 year were the only independent predictors for PCP diagnosis in lung cancer patients suspected of PCP. See Table 2.
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Age | 1.163 (1.034, 1.309)a | 0.004 |
| NSCLC—Stage 3 | 2.993 (0.552, 16.24) | 0.202 |
| Absolute neutrophils count (103/µL) | 1.12 (0.986, 1.272) | 0.079 |
| Radiotherapy within 1 year | 5.831 (1.333, 25.504) | 0.017 |
| Chemotherapy within 3 months | 2.684 (0.564, 12.769) | 0.198 |
- a OR for variable AGE given for 10 additional years.
- Abbreviation: NSCLC, non-small-cell lung cancer.
3.3 Description of PCP Diagnosed Patients and Determinants of Mortality
Patients diagnosed with PCP were treated mainly with co-trimoxazole (50 patients, 92.6%) and 40 (74.1%) patients required oxygen support. CAT was given in 29 (55.2%) patients, for a median duration of 21 days (IQR 6–21). Twenty-three (42.6%) patients were admitted in the ICU, and the primary outcome 21-day mortality occurred in 17 (35.4%), while 6 were lost to follow-up before Day 21. All patients had abnormal chest X-ray, and high-resolution chest tomography was practiced in 34 (63%) patients, results are shown in Table S2.
In patients diagnosed with PCP, background disease was found to be associated with 21-day mortality, with hematological malignancy being a risk factor (OR 4.481; 95% CI 1.313–16.791; p = 0.020). Mortality at Day 21 was significantly less in the subgroup of patients with solid cancer other than lung cancer compared with other underlying disease (p = 0.005), with no death occurring in this subgroup. There was a nonsignificant trend toward higher mortality in older patients (p = 0.062). Baseline absolute neutrophil count (ANC) showed negative association with 21-day mortality (OR 0.829; 95% CI 0.693–0.947, p = 0.017), and ANC lower than 4.103/µL was associated with a 4.62 OR for mortality at Day 21 (p = 0.023). Radiological features were not significantly associated with 21-day mortality. There was a trend toward an association between viral coinfection and higher mortality (OR 3.640; 95% CI 0.951–15.040; p = 0.063). CT value was not associated with any baseline variable, nor with any outcome. CAT was not associated with any outcome. As expected, 21-day mortality was strongly associated with oxygen requirement (p = 0.005) and ICU admission (OR 16.0; 95% CI 3.961–85.735, p < 10−4) (see Table 3). The results of analysis for risk factor of ICU admission in PCP diagnosed patients are presented in Table S3. The risk factors appear to be similar to those for mortality on Day 21.
| Descriptive statistics | Univariate analysis | |||||
|---|---|---|---|---|---|---|
| All PCP patients | Alive at Day 21 | Dead at Day 21 | ||||
| n | 54 | 31 | 17 | p value | OR (95% CI) | p value |
| Demographics | ||||||
| Age (years) | 63.22 [56.01, 70.38] | 63.17 [55.02, 67.26] | 70.69 [58.92, 74.67] | 0.062 | 1.045 (0.995–1.112) | 0.114 |
| Sex (male) | 40 / 54 (74.1%) | 19 / 31 (61.3%) | 15 / 17 (88.2%) | 0.103 | 4.737 (1.074–33.532) | 0.064 |
| Background disease | ||||||
| Hematological malignancy | 22 / 54 (40.7%) | 9 / 31 (29.0%) | 11 / 17 (64.7%) | 0.036 | 4.481 (1.313–16.791) | 0.020 |
| AML | 2 / 54 (3.7%) | 1 / 31 (3.2%) | 1 / 17 (5.9%) | 1.000 | 1.875 (0.071–49.568) | 0.664 |
| ALL | 4 / 54 (7.4%) | 2 / 31 (6.5%) | 2 / 17 (11.8%) | 0.927 | 1.933 (0.215–17.456) | 0.530 |
| NHL | 8 / 54 (14.8%) | 4 / 31 (12.9%) | 3 / 17 (17.6%) | 0.986 | 1.446 (0.255–7.473) | 0.657 |
| HL | 3 / 54 (5.6%) | 0 / 31 (0.0%) | 3 / 17 (17.6%) | 0.073 | ||
| MM | 5 / 54 (9.3%) | 2 / 31 (6.5%) | 2 / 17 (11.8%) | 0.927 | 1.933 (0.215–17.456) | 0.530 |
| NMHD | 2 / 54 (3.7%) | 0 / 31 (0.0%) | 2 / 17 (11.8%) | 0.232 | ||
| Solid cancer | 30 / 54 (55.6%) | 22 / 31 (71.0%) | 4 / 17 (23.5%) | 0.004 | 0.126 (0.029–0.459) | 0.003 |
| Lung cancer | 14 / 54 (25.9%) | 9 / 31 (29.0%) | 4 / 17 (23.5%) | 0.944 | 0.752 (0.175–2.831) | 0.682 |
| Other solid cancer | 16 / 54 (29.6%) | 13 / 31 (41.9%) | 0 / 17 (0.0%) | 0.005 | ||
| Lung metastasis | 6 / 54 (11.1%) | 2 / 31 (6.5%) | 3 / 17 (17.6%) | 0.471 | 3.107 (0.465–25.698) | 0.242 |
| Positive HIV serology | 2 / 46 (4.3%) | 1 / 27 (3.7%) | 0 / 15 (0.0%) | 1.000 | ||
| Treatments previously received | ||||||
| HSCT | 3 / 54 (5.6%) | 1 / 31 (3.2%) | 2 / 17 (11.8%) | 0.585 | 4.000 (0.356–90.225) | 0.273 |
| Chemotherapy within 1 month | 27 / 54 (50.0%) | 20 / 31 (64.5%) | 5 / 17 (29.4%) | 0.043 | 0.263 (0.069–0.898) | 0.039 |
| High dose steroids exposure | 2 / 54 (3.7%) | 0 / 31 (0.0%) | 2 / 17 (11.8%) | 0.232 | ||
| PCP prophylaxis | 0 / 53 (0.0%) | |||||
| Biological features at baseline | ||||||
| LDH (U/L) | 404.00 [275.00, 596.00] | 526.00 [374.25, 706.50] | 434.00 [351.25, 540.50] | 0.722 | 1.001 (1–1.004) | 0.567 |
| Procalcitonin (ng/mL) | 0.46 [0.27, 1.18] | 0.73 [0.31, 1.39] | 0.27 [0.24, 0.48] | 0.231 | 1.029 (0.967–1.125) | 0.399 |
| Lymphocytes (103/µL) | 0.70 [0.40, 1.10] | 0.70 [0.30, 1.10] | 0.50 [0.40, 0.70] | 0.436 | 0.802 (0.341–1.508) | 0.537 |
| ANC (103/µL) | 6.55 [3.92, 10.75] | 8.00 [4.50, 14.80] | 4.80 [2.10, 6.60] | 0.010 | 0.829 (0.693–0.947) | 0.017 |
| WBC (103/µL) | 9.30 [5.50, 13.17] | 9.60 [5.90, 16.05] | 7.20 [3.90, 10.50] | 0.030 | 0.889 (0.779–0.984) | 0.049 |
| Sample used for Pneumocystis jirovecii PCR | ||||||
| Sputum | 28 / 54 (51.9%) | 21 / 31 (67.7%) | 4 / 17 (23.5%) | 0.009 | 0.147 (0.034–0.528) | 0.005 |
| ETS | 11 / 54 (20.4%) | 2 / 31 (6.5%) | 7 / 17 (41.2%) | 0.010 | 10.15 (2.065–76.254) | 0.009 |
| BAL | 15 / 54 (27.8%) | 8 / 31 (25.8%) | 6 / 17 (35.3%) | 0.719 | 1.568 (0.425–5.676) | 0.491 |
| Coinfection | 26 / 54 (48.1%) | 13 / 31 (41.9%) | 10 / 17 (58.8%) | 0.413 | 1.978 (0.602–6.805) | 0.266 |
| Fungal | 1 / 54 (1.9%) | 0 / 31 (0.0%) | 1 / 17 (5.9%) | 0.758 | ||
| Bacterial | 14 / 54 (25.9%) | 9 / 31 (29.0%) | 4 / 17 (23.5%) | 0.944 | 0.752 (0.175–2.831) | 0.682 |
| Viral | 14 / 54 (25.9%) | 5 / 31 (16.1%) | 7 / 17 (41.2%) | 0.117 | 3.64 (0.951–15.04) | 0.063 |
| Sars-Cov-2 | 4 / 53 (7.5%) | 3 / 31 (9.7%) | 1 / 17 (5.9%) | 1.000 | 0.583 (0.028–4.998) | 0.652 |
| Influenza | 3 / 53 (5.7%) | 0 / 31 (0.0%) | 3 / 17 (17.6%) | 0.073 | ||
| PCP treatment | ||||||
| Cotrimoxazole | 50 / 54 (92.6%) | 30 / 31 (96.8%) | 15 / 17 (88.2%) | 0.585 | 0.25 (0.011–2.81) | 0.273 |
| Clindamycin + primaquine | 2 / 54 (3.7%) | 0 / 31 (0.0%) | 1 / 17 (5.9%) | 0.758 | ||
| CAT | 29 / 52 (55.8%) | 14 / 29 (48.3%) | 11 / 17 (64.7%) | 0.439 | 1.964 (0.584–7.068) | 0.283 |
| CAT dosage (mg/day, eq prednisone) | 56.50 [32.50, 100.00] | 50.00 [37.50, 85.00] | 63.00 [22.50, 100.00] | 1.000 | 1.002 (0.979–1.027) | 0.859 |
| CAT duration (days) | 21.00 [6.00, 21.00] | 21.00 [9.00, 21.00] | 3.00 [3.00, 3.00] | 0.105 | ||
| Oxygen support | 40 / 54 (74.1%) | 18 / 31 (58.1%) | 17 / 17 (100.0%) | 0.005 | ||
| Mechanical ventilation | 27 / 54 (50.0%) | 9 / 31 (29.0%) | 15 / 17 (88.2%) | < 0.001 | 18.333 (4.114–132.815) | 0.001 |
| Outcomes | ||||||
| ICU admission | 23 / 54 (42.6%) | 7 / 31 (22.6%) | 14 / 17 (82.4%) | < 0.001 | 16 (3.961–85.735) | < 10−4 |
| ICU LOS (days) | 0.00 [0.00, 5.50] | 0.00 [0.00, 0.00] | 4.00 [2.00, 8.00] | 0.001 | 1.051 (0.968–1.156) | 0.247 |
| In ICU death | 12 / 54 (22.2%) | 1 / 31 (3.2%) | 11 / 17 (64.7%) | < 0.001 | 55 (8.476–1109.145) | < 10−4 |
| In hospital LOS (days) | 11.00 [7.00, 16.00] | 9.00 [6.00, 13.00] | 11.00 [7.00, 16.00] | 0.650 | 0.986 (0.935–1.023) | 0.511 |
| In hospital death | 21 / 53 (39.6%) | 4 / 30 (13.3%) | 17 / 17 (100.0%) | < 0.001 | ||
| Lost to follow-up | 6 / 54 (11.1%) | |||||
| Mortality at Day 21 | 17 / 48 (35.4%) | 0 / 31 (0.0%) | 17 / 17 (100.0%) | |||
- Abbreviations: ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; ANC, absolute neutrophil count; BAL, bronchoalveolar lavage; CAT, corticosteroid adjuvant therapy; ETS, endotracheal secretion; HIV, human immunodeficiency virus; HL, Hodgkin lymphoma; HSCT, hematopoietic stem cells transplant; ICU, intensive care unit; LDH, lactate dehydrogenase; LOS, length of stay; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; NMHD, nonmalignant hematological disease; PCP, Pneumocystis jirovecii pneumonia; WBC, white blood count.
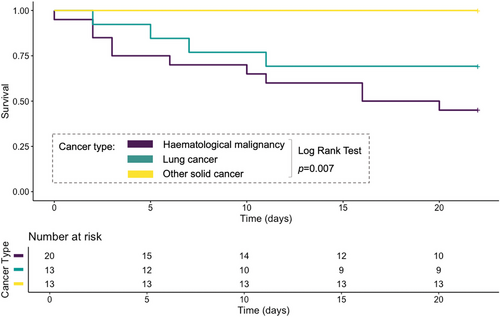
Multivariate logistic regression analysis included age, background hematological malignancy, chemotherapy within 1 month, and ANC at baseline. Age and hematological malignancy appeared to be independent risk factors for 21-day mortality, whereas higher ANC at baseline and a history of chemotherapy within 1 month appeared to be independently associated with better outcomes (see Table 4). Survival analysis were conducted stratified on three subsets of background disease: hematological malignancy, lung cancer, other nonlung solid cancer. The three subsets showed 70.6%, 23.5%, and 0.0% 21-day mortality, respectively. Analysis by log-rank test proved the survival curves to be statistically different (p = 0.007) (see Figure 1).
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Age | 1.134 (1.017, 1.265)a | 0.002 |
| Hematological malignancy | 19.855 (1.765, 223.319) | 0.002 |
| Chemotherapy within 1 month | 0.098 (0.013, 0.722) | 0.009 |
| Absolute neutrophils count (103/µL) | 0.793 (0.642, 0.979) | 0.007 |
- a OR for variable AGE given for 10 additional years.
4 Discussion
Our study results highlight the high mortality of PCP in cancer patients, especially in those with hematological malignancy.
Older age appears as a key risk factor for both occurrence and lethality of PCP, in all subgroups. This finding is consistent with previous studies conducted in cancer and noncancer patients [14, 15]. Therefore, high suspicion of PCP should prevail in older cancer patients with atypical pneumonia.
Our results also highlights the efficacy of prophylaxis, as no patient on prophylaxis developed PCP in our cohort. Prophylaxis was given only to hematological malignancy patients. No patient fulfilled the criteria for prolonged corticosteroid therapy requiring PCP prophylaxis as per guidelines. Our study was not designed to assess appropriate use of PCP prophylaxis, but the results seem to reflect a good adhesion to international guidelines and local protocols. Still, we observed that some hematological malignancy at-risk patients were not on prophylaxis and developed PCP: This probably reflects the real-life difficulties of PCP prophylaxis, as co-trimoxazole can be associated with poor tolerance or contraindications [16]. Atovaquone as well as pentamidine are not widely available in India.
Our focus on the subgroup of lung cancer patients provides interesting insights, despite its small size. First, PCP appears to be a lethal disease in those patients, Jul 18;(3): with 30.8% mortality at Day 21. Despite this high mortality rate, no prophylaxis was recommended in our lung cancer patients according to the current guidelines, as none received high-dose long duration corticosteroid [5]. Second, in our cohort of lung cancer patients, older age and a history of radiotherapy within 1 year appeared to be strong risk factors for PCP. The risk of PCP in patients with history of radiotherapy had been highlighted by others, and in these studies, radiotherapy was associated with chemotherapy or concomitant high-dose steroid exposure [2, 17]. In our study radiotherapy seems to be an independent risk factor for occurrence of PCP. In contrast, history of treatment with newer targeted therapies such as tyrosine kinase inhibitors or check-point inhibitor immunotherapy was not associated with PCP diagnosis. This is in line with existing data and pathophysiological understanding, as these drugs do not target the pathways of immune response against P. jirovecii [18]. Although Stage 3 lung cancer was associated with increased risk of PCP in univariate analysis, this association disappeared after stratification over history of radiotherapy within 1 year, indicating that Stage 3 association with PCP diagnosis is mainly related to the radiotherapy exposure in these patients. Previous study by Lee et al. in cancer patients did not find a relation between stage of the disease and PCP [2]. Altogether, this result should increase awareness of clinicians regarding the possibility of PCP in lung cancer patients with recent history of radiation therapy, as rapid diagnosis and treatment is a key factor for better survival [19]. The high lethality and significant incidence of PCP in lung cancer patients highlights the need to revisit the current recommendations of not using PCP prophylaxis in adults with solid tumors. This question has already been raised by other teams and for children [7, 20], but further studies are needed to better assess the at-risk population and to guide future recommendation regarding PCP prophylaxis.
Independent risk factors for mortality in PCP patients were old age and background disease, with a very high odds ratio of 128.8 for hematological malignancy. Among solid cancers, lung cancer patients appeared to be at much higher risk of death compared with other solid cancer patients. This is in line with recent data showing a 100% increased risk of mortality in lung cancer patients compared with other solid cancer patients, when diagnosed with PCP [21]. We want to discuss two more original and intriguing findings in our work. Firstly, recent chemotherapy being a protective factor for mortality seems contra intuitive as it is an additional immunosuppression factor. We hypothesize that chemotherapy within 1 month is a marker of fitness and infection-free status, more than a protective factor. Stratification of the patients over a variable representing fitness such as performance status could have answered this hypothesis, but no such variable has been collected. The second original and intriguing finding is the protective role of higher ANC on mortality. Moreover, this relation appears to be independent of the use of chemotherapy or the type of malignancy. The role of lymphocytes in PCP pathophysiology is well understood, but neutrophils do not appear to play a major role [22]. Neutrophils play a role in immune response against concomitant bacterial infections, but ANC association with mortality at 21 days showed no interactions in bivariate analysis with the following variables: bacterial, viral, or fungal coinfection, and procalcitonin level. Thus, it remains uncertain how to interpret this association between mortality and ANC. Also, our study was not designed to assess immunological parameters with precision, and measurement of parameters such as CD4 lymphocytes count or CD4/CD8 ratio might have provided interesting insights over the immune profile of our patients. As expected, given our small sample size, CAT was not statistically associated with any outcome.
Limitations of our study are related to its retrospective and observational design, and to the small number of patients diagnosed with PCP. Another limitation is the definition of PCP, based on the combination of clinical suspicion, compatible chest X-ray, and P. jirovecii PCR result. Given the excellent sensitivity of this result and the concerns about colonization and false positives with PCR, it is possible that some patients were misclassified as PCP-positive patients. However, most patients in the PCP group had concomitant compatible findings on HRCT and were treated accordingly. Thus, these possibly misclassified patients would reflect the real-life uncertainties in diagnosing PCP in non-HIV immunocompromised patients and would not be a significant drawback for this study. Also, our institution did not use routine direct examination nor Beta-D-Glucan serology at the time of study. This reflects a real-life situation in an era where molecular diagnosis is more widely used than Beta-D-Glucan and staining. Another limitation is the absence of definitive diagnosis regarding the etiology of respiratory disease in the control group, making it difficult to compare the outcomes of PCP-positive and PCP-negative patients. Finally, due to the design of our cohort, the risk factors found in this study should be interpreted as risk factors of PCP diagnosis in patients clinically suspected of PCP only.
Our study has several strengths: (i) provides a comprehensive overview of PCP cases in a tertiary cancer center in a developing country, (ii) physician-centered design, addressing real-life questions such as identification of at-risk patients for developing or dying of PCP, (iii) focus on lung cancer patients, an overlooked population with high PCP lethality.
5 Conclusion
PCP in cancer patients is a lethal disease, and at-risk groups for developing PCP also appeared at high risk of fatal evolution in case of PCP. Prophylaxis had complete efficacy in our study, thus compliance to prophylaxis recommendations appears as the key to minimize the burden of PCP in cancer settings. Physicians awareness should be raised regarding neglected groups of at-risk patients such as older cancer patients and lung cancer patients with history of radiotherapy, to allow prompt diagnosis and treatment.
Acknowledgments
During the preparation of this work, the author V. Franchi used ChatGPT by OpenAI in order to check for grammar and spelling. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




